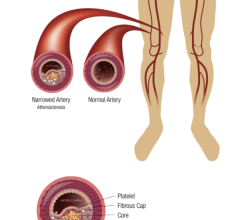
August 14, 2012 — The U.S. Food and Drug Administration (FDA) is hosting a public workshop that examines use of absorbable materials in implantable devices for endovascular therapies such as fully absorbable cardiovascular stents, where the stent platform degrades, as well as absorbable coatings.
The workshop is entitled "ASTM-FDA Workshop on Absorbable Medical Devices: Lessons Learned from Correlations of Bench Testing and Clinical Performance." It is scheduled for 8:30 a.m., Nov. 28, 2012, at the FDA White Oak Campus, 10903 New Hampshire Ave., Building 31 Conference Center (Great Room, Room 1503), Silver Spring, Md.
Recent studies have identified promising results for the use of absorbable materials in implantable devices. The use of these materials for cardiovascular indications poses new risks due to the critical fatigue and mechanical loading demands that the implant must withstand and perform. However, the optimal preclinical/bench-testing paradigm to predict clinical performance of fully absorbable cardiovascular devices is not yet defined.
This workshop will discuss the use of absorbable materials (including synthetic polymers as well as erodible metals) in medical devices across a broad range of indications with the aim of defining successful and unsuccessful methods to predict clinical performance, and will subsequently apply these methods to unique challenges for cardiovascular indications. Presenters are invited to share their experience from cardiovascular and non-cardiovascular medical devices, as well as devices that are fully absorbable, and devices with only a component or coating that is absorbable. This workshop will bring together the expertise of academia and industry professionals to define test methods as well as to educate and inform their colleagues in industry, academia and device regulation on the performance and predictability of absorbable medical device degradation. Workshop participants will seek to define the critical factors for preclinical/bench testing and clinical predictability. They will then apply lessons learned from marketed devices for non-cardiovascular indications to the emerging uses of absorbable devices to treat cardiovascular disease.
Topics to be discussed at the workshop include:
- Correlations of in vitro and in vivo absorption;
- Quantitative characterization of absorption kinetics;
- Test methods to identify interactions of absorption with mechanical loading; and
- Test methods to assess mechanical performance of the absorbable product.
The lessons learned from both early cardiovascular and well-established non-cardiovascular device experiences will be presented. These lessons will be discussed in the context of emerging cardiovascular uses of absorbable materials as part of a panel session at the end of the workshop.
FDA is co-sponsoring the workshop together with ASTM International, an organization responsible for the development and delivery of international voluntary consensus standards.
The purpose of the workshop is to provide a forum for industry, academia and the FDA to discuss test methods for establishing correlations between in vitro and in vivo degradation of absorbable implant devices, and the interaction of mechanical loading and mechanical performance with degradation. While there will be an emphasis on cardiovascular indications as part of a panel session, characterization techniques and experiences from both cardiovascular as well as non-cardiovascular devices will be discussed and are encouraged.
There is no fee to register for the workshop and registration will be on a first-come, first-served basis. Early registration is recommended because seating is limited.
To submit an abstract for consideration to be presented, please visit www.astm.org/f04wkshp1112.htm.
For more information: [email protected] or [email protected]

 May 30, 2024
May 30, 2024