August 15, 2012 — Sound Interventions Inc. has released three-month data from the company's first-in-human clinical study (SOUND-ITV) to treat resistant hypertension through the use of catheter-based ultrasound.
In addition to office-based blood pressure measurements, patients in the study underwent 24-hour ambulatory blood pressure monitoring (ABPM) prior to the ultrasonic renal denervation procedure, and subsequently three months after the procedure. The office-based blood pressure measurement results showed an average decrease of -25.6/-12.5 mm Hg. Consistent with these results, the 24-hour mean blood pressure decreased by -23.1/-11.9 mm Hg.
Patients were treated at Holmolka Hospital in Prague, Czech Republic. Patients enrolled in the study were selected based on a history of hypertension that could not be controlled with medical therapy. The SOUND-ITV study was designed to evaluate the safety and effectiveness of the company's volumetric dosimetry-controlled application of unfocused ultrasound (patents pending).
"These results demonstrate the ability of Sound Interventions' ultrasound technology to significantly lower blood pressure in patients whose blood pressure was unable to be controlled by conventional pharmaceutical therapy," said Dr. Petr Neuzil, CSc., FESC, chairman, department of cardiology, Holmolka Hospital.
"The use of 24-hour monitoring to assess results of renal denervation procedures is a more accurate measurement of effectiveness than office-based blood pressure. The favorable results seen on the ABPM fortify our confidence in the efficacy of this technology," said Vivek Reddy, Mount Sinai Medical Center, New York City, who collaborated with Neuzil and is an advisor to Sound Interventions.
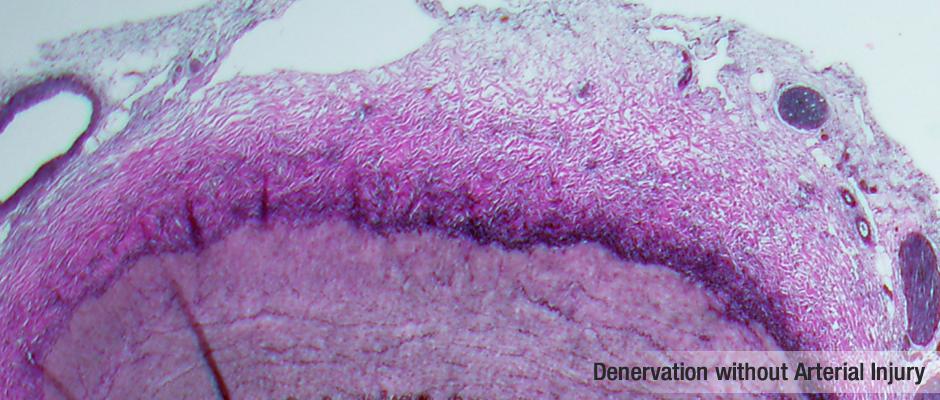
"These results demonstrate the efficacy of Sound Interventions' ultrasound dosimetry-controlled approach to renal denervation. Prior to initiation of the SOUND-ITV study, the company performed extensive in vitro and in vivo testing in order to perfect the ultrasound dosimetry. The preclinical testing has demonstrated that the Sound Interventions technology is unique in its ability to ablate the target nerve fibers while sparing the arterial wall," said David Smith, president and CEO of Sound Interventions. "The success of the acute procedures in the SOUND-ITV study, along with these very strong clinical results, validate this research and indicate that we are well on the way to developing the state-of-the-art renal denervation technology."
Results of the SOUND-ITV study will be presented at the upcoming 2012 TCT conference in Miami Beach, Fla., Oct. 22-26, 2012.
For more information: www.sounditv.com


 January 05, 2026
January 05, 2026 









