May 27, 2014 — Terumo BCT has entered into a business relationship with Kaneka to gain market authorization in the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
May 23, 2014 — Stentys presented final results from the APPOSITION IV study of its new self-apposing sirolimus-eluting ...
May 23, 2014 — The first drug-eluting balloon to go up for review by the U.S. Food and Drug Administration (FDA) will be ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
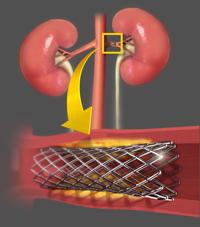
May 21, 2014 — Patients with hypertension after renal artery stenting who do not respond to drug treatment may have ...
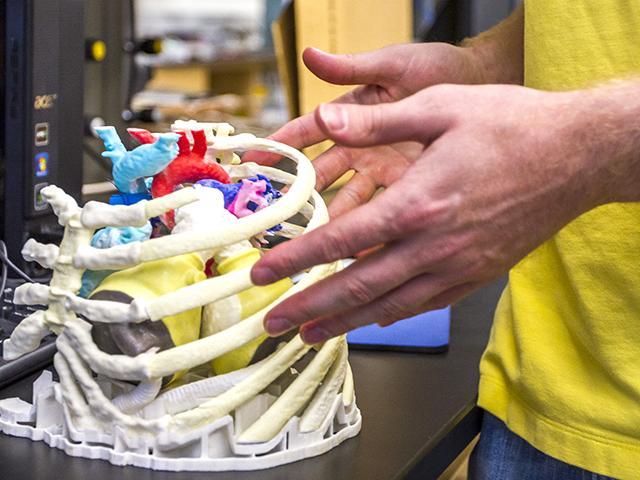
May 20, 2014 — It is possible to use 3-D printer technology to create some types of customized medical devices on demand ...
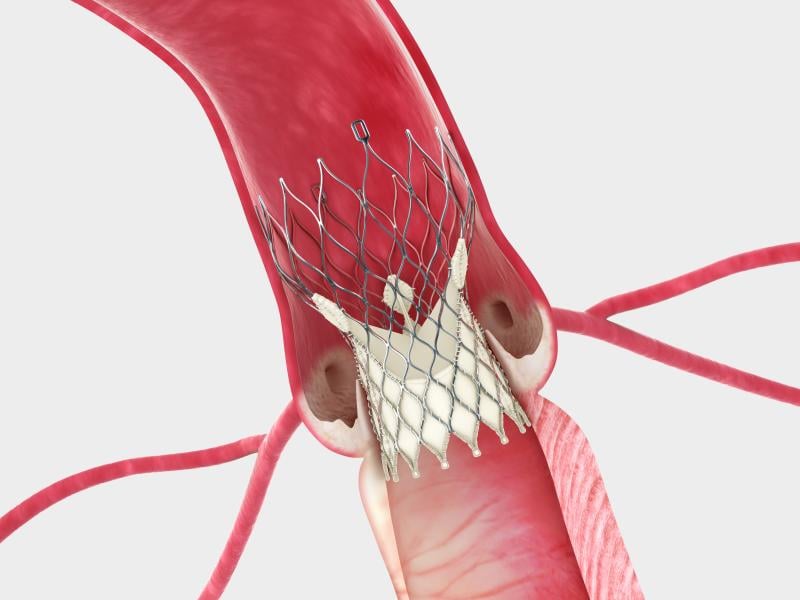
May 20, 2014 — In a surprise move, Edwards Lifesciences Corp. and Medtronic reached an agreement this week to settle all ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

(Editors note: This article was written in 2014, and was updated with more recent information in January 2017.)
Compu ...
May 16, 2014 — In a move to expand significantly its portfolio of solutions for peripheral interventions, Boston ...
May 15, 2014 — CathMaps demonstrated how mobile technology can provide emergency assistance and peace of mind to ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 14, 2014 — OrbusNeich announced the launch of the Sapphire II NC coronary dilatation catheter, a non-compliant ballo ...
May 14, 2014 — Detroit Medical Center (DMC) Harper Hospital presented a live CorPath robotic-assisted percutaneous ...

May 13, 2014 — Elixir Medical Corp. received European CE mark approval for its DESolve 100 Novolimus-eluting Bioresorbab ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 9, 2014 — InspireMD Inc. initiated a Voluntary Field Action (VFA) following recent reports of MGuard Prime EPS ...
May 8, 2014 — Argon Medical Devices Inc. began marketing the CelanerXT rotational thrombectomy system as a new addition ...
May 7, 2014 — Women face greater limits on their lifestyle and have more severe symptoms as a result of peripheral ...

 May 27, 2014
May 27, 2014






