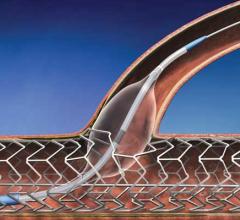
November 17, 2014 — VIVA Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Nov. 14, 2014 — Covidien releases 12-month results of the DEFINITIVE AR study, the first randomized study designed to ...
Nov. 14, 2014 — Micell Technologies Inc. announced commercialization plans for its MiStent Sirolimus Eluting Absorbable ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Nov. 13, 2014 — Optimizing the treatment of coronary artery disease with a new foundation for future stent innovations ...
Nov. 12, 2014 — Catalist-listed QT Vascular Ltd. has acquired a novel technology platform called Java, and all ...
November 11, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 11, 2014 — TIDI Products LLC has acquired CFI Medical Solutions (CFI) of Fenton, Mich., a diversified medical ...

Nov. 11, 2014 — Viva Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
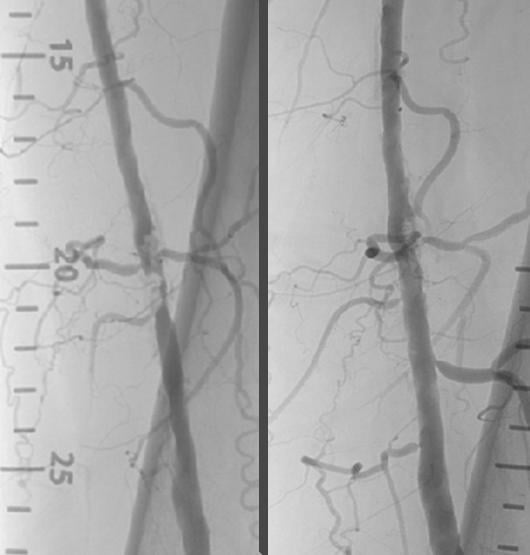
Nov. 11, 2014 — Shockwave Medical announced positive clinical results from Disrupt PAD trial, a single-arm multicenter ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Nov. 10, 2014 — For the treatment of peripheral artery disease in leg arteries above the knee, the IN.PACT Admiral drug ...
Nov. 10, 2014 — Merit Medical Systems Inc. settled a dispute regarding the distribution of the SecureLoc Safety ...
November 6, 2014 — Cardinal Health announced that its MynxGrip Vascular Closure Device recently received U.S. Food and ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Nov. 6, 2014 — Cardiovascular Systems Inc. received the CE Mark for its Stealth 360º Orbital Atherectomy System (OAS) ...
Nov. 5, 2014 — Food and Drug Administration gave 510(k) clearance for the HawkOne directional atherectomy system. The ...
November 4, 2014 — Shockwave Medical announced that the company will present clinical results from DISRUPT PAD, a single ...

 November 17, 2014
November 17, 2014