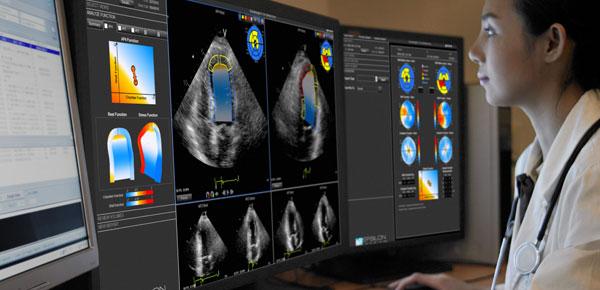
Below is a round up of news and videos about new cardiac ultrasound technology discussed in sessions and on the expo ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
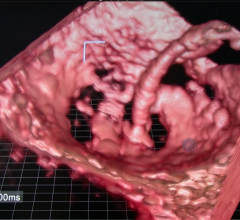
June 30, 2016 — Vital Images Inc. recently launched version 7 of its Vitrea advanced visualization software. This ...
June 30, 2016 — BioVentrix Inc. announced that it has received certification for CE marking its Revivent TC ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Interview with Stephen Little, M.D., medical director of the Valve Clinic at the Houston Methodist DeBakey Heart and ...
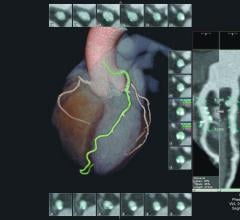
June 29, 2016 — HeartFlow Inc. announced that it is launching its next generation of the HeartFlow FFR-CT Analysis. The ...
Interview with Rebecca Hahn, M.D., FASE, Columbia University Medical Center, New York, at the American Society of ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 21, 2016 — Zoll Medical Corp. announced that patients experience a high one-year survival rate following use of the ...
This is an animation, supplied by Gore, demonstrated how the Cardioform Septal Occluder is implanted for the ...
I saw a new combination of technologies on the expo floor at the American Society of Echocardiography (ASE) meeting in ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 13, 2016 — GE Healthcare and Getinge Group announced the U.S. launch of a new, highly flexible angiography solution ...
June 8, 2016 — The amount a heart ‘bleeds’ following a heart attack can predict the severity of future heart failure ...
June 7, 2016 — Intact Vascular Inc. announced that its Tack Optimized Balloon Angioplasty III (TOBA III) clinical trial ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 6, 2016 — Amaranth Medical announced that it completed enrollment in May in the RENASCENT-II study of its novel ...
June 3, 2016 — Biotronik announced results establishing non-inferiority of the Orsiro hybrid drug-eluting stent (DES) to ...
June 3, 2016 — Cardionovum GmbH recently announced the completion of enrollment of the RAPID trial. Results will be used ...

 July 01, 2016
July 01, 2016