January 9, 2017 — BioCardia Inc. announced in December the issuance of United States Patent No. 9,517,199 relating to a ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
January 9, 2017 — Whale Imaging Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the G-Arm Duo ...
ITN and DAIC Editor Dave Fornell takes a tour of some of the most innovative new technologies being displayed on the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 5, 2017 — A new report published by Allied Market Research forecasts that the global thrombectomy devices market ...
January 5, 2017 — Abbott announced it completed the acquisition of St. Jude Medical Inc. The transaction provides Abbott ...
January 4, 2017 — A new minimally invasive technique for repairing the most common cardiac birth defect in extremely ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 4, 2015 — At RSNA 2016, Siemens Healthineers unveiled its 510(k)-pending robot-supported Artis pheno angiography ...
January 3, 2017 — The Impella CP heart pump (Abiomed) demonstrated no improvement in mortality for patients with ...
This video, provided by Valtech, demonstrates the Cardioband transcatheter mitral annuloplasty system. It allows ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 2, 2017 — The U.S. Food and Drug Administration (FDA) hopes to build clinical evidence through new registries ...
December 31, 2016 — NaviGate Cardiac Structures Inc. (NCSI) announced that a novel valved stent that can capture the ...
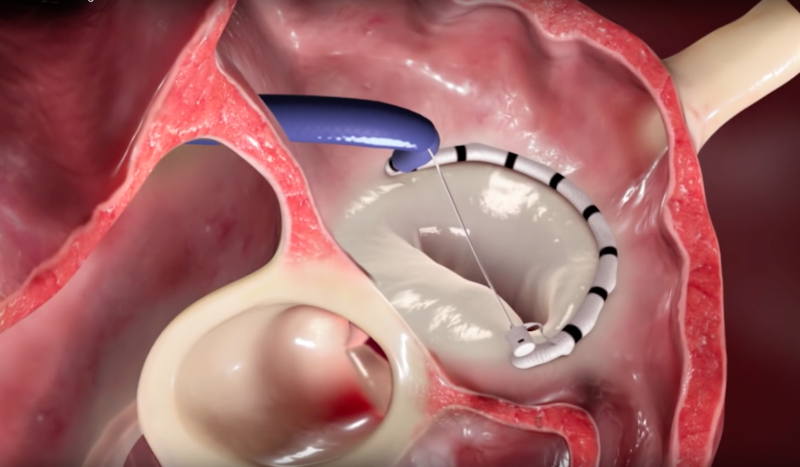
The Cardioband System (Valtech) has recently been introduced as a potential option for transcatheter repair of the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
As wearable and implantable patient monitoring or therapy devices become more sophisticated with advanced wireless ...
December 29, 2016 — The American College of Cardiology (ACC), along with several partnering organizations, recently ...
December 29, 2016 — The Department of Health and Human Services (HHS) finalized new Medicare alternative payment models ...

 January 09, 2017
January 09, 2017