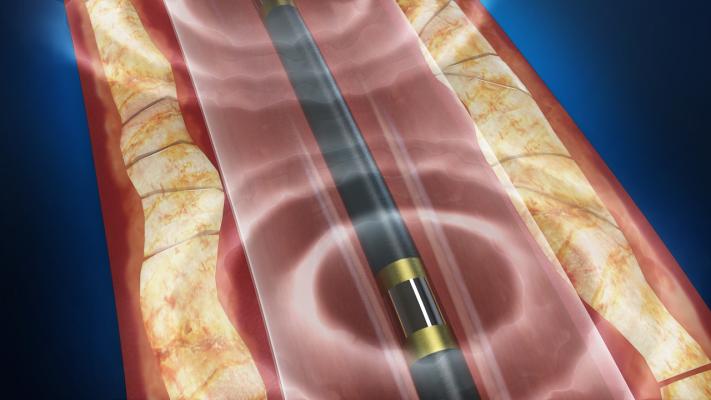
The Shockwave Medical Lithoplasty system combines a balloon catheter with tiny lithotripsy electrodes that emit pulses to crack and break up calcium in peripheral arteries.
September 16, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance for Shockwave Medical’s Lithoplasty System for the treatment of calcified plaque in patients with peripheral artery disease (PAD). The technology is the first of its kind in interventional cardiovascular medicine, using similar lithotripsy technology already employed to break up kidney stones. The technology uses a balloon catheter containing miniature lithotripsy electrodes that crack and break up calcium in vessel walls, without the need to overinflate the balloon, which can lead to extensive vessel damage.
Watch an animation of how the Lithoplasty System works
PAD, affecting nearly 9 million people in the U.S.,[1] blocks blood flow to the legs and feet, causing significant pain and limited mobility, potentially leading to surgery or even amputation in severe cases. Arterial calcification, caused by plaque that hardens over time, is increasingly common as preventive care and disease management have enabled patients to live longer, making vascular disease a chronic condition. In fact, over half of all patients with peripheral vascular disease have moderate or severe calcification in their arteries.[2] Limitations of currently available interventional devices make successful treatment of patients with calcified arteries increasingly more difficult. The Shockwave Medical Lithoplasty System is designed to overcome those limitations.
The Lithoplasty System is the first-ever device designed to selectively target hardened calcium in patients with cardiovascular disease. The device integrates two familiar technologies: the calcium-disrupting power of sound waves using lithotripsy with an angioplasty balloon. Intermittent lithotripsy pulses disrupt both superficial and deep vascular calcium while minimizing soft tissue injury, and an integrated angioplasty balloon expands blockages at low pressures to restore blood flow.
“Lithoplasty represents a new mechanism of treatment and is revolutionary for the care of patients with calcified peripheral vascular disease, a difficult-to-treat patient population,” said Kenneth Rosenfield, M.D., section head for vascular medicine and intervention at Massachusetts General Hospital, Boston. “Existing devices for treating these patients have significant shortcomings that make it challenging to successfully open arteries while minimizing vascular injury and complications. Lithoplasty is a unique approach that allows us to successfully treat these diseased vessels using a device built on a familiar balloon catheter platform, while minimizing the risk of vessel injury, including dissections that require stenting or other additional interventions.”
“This marks an exciting milestone for the company as we prepare to begin our commercial activities in the United States,” said Shockwave Medical CEO and co-founder Daniel Hawkins. "We view this as an important validation of our technology's potential to address the burdens of vascular calcification, and we are looking forward to working with the clinical community to deeply integrate Lithoplasty into the care pathway to improve outcomes for patients with advanced cardiovascular diseases.”
Watch the VIDEO “Breaking Up Calcified Lesions Without Vessel Trauma.” A discussion with Todd Brinton, M.D., at TCT 2016 about the newly FDA-cleared Shockwave Medical Lithoplasty System. Brinton is clinical associate professor and adjunct associate professor of bioengineering at Stanford University Medical Center.
Clinical Data
Positive data from the DISRUPT PAD Study, a single-arm, two-phase, multicenter study evaluating the safety and performance of the Shockwave Medical Lithoplasty System to treat peripheral artery disease, supported the clearance. Clinical data from DISRUPT PAD demonstrated compelling safety, consistent procedural success across all patient subgroups, significant and immediate increases in blood flow in treated vessels, and minimal vessel injury. The latest clinical results from the DISRUPT PAD studies will be presented at the Vascular Interventional Advances (VIVA) conference Sept. 19, 2016 in Las Vegas.
“We are thrilled to reach this important milestone and we look forward to partnering with physicians to help advance the treatment of peripheral artery disease,” said Brinton, who is also a co-founder of Shockwave Medical.
The company plans a limited U.S. commercial release of the Lithoplasty System in 2017 and will initiate a global randomized trial to gather further clinical data on the benefits of Lithoplasty treatment.
For more information: www.shockwavemedical.com
References:
1. Roger VL, Go AS, Lloyd-Jones DM, et. al. “Heart Disease and Stroke Statistics 2011 Update: A Report from the American Heart Association.” Circulation 2011;123:e18-e209.
2. Rocha-Singh KJ, Zeller T, Jaff MR. “Peripheral arterial calcification: prevalence, mechanism, detection and clinical implications.” Catheterization and Cardiovascular Interventions 2014; 83:E212-E220


 June 13, 2024
June 13, 2024 









