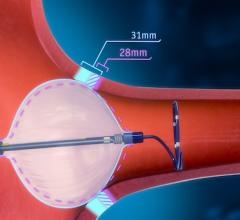
There is a movement toward real-time, remote cardiac monitoring with the latest generation of event and Holter monitor ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
June 12, 2018 — Volta Medical, out of Marseille, France, has developed the first artificial intelligence (AI) software ...
June 5, 2018 — Philips Healthcare has signed an agreement to acquire EPD Solutions, a provider of image-guidance in ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

June 5, 2018 — The future of cardiovascular care will be transformed by advances in artificial intelligence, digital ...
June 1, 2018 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
May 30, 2018 — Data from the GENETIC-AF trial was presented in a “Late Breaking Clinical Trials” oral presentation at ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
May 25, 2018 — Data from the first-in-human study using pulsed electric field (PEF) energy ablate heart tissue in the ...
May 24, 2018 — Atrial fibrillation patients who are diagnosed with carotid artery disease face higher risks for ...
New patient monitoring technologies with wireless connectivity have enabled a revolution in cardiac event and Holter ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
May 22, 2018 — Medtronic plc announced study results showing its AdaptivCRT algorithm is associated with improved ...
May 22, 2018 — LuxCath LLC showcased the next generation of its proprietary OmniView light-guided catheter ablation ...
May 21, 2018 — Complete pulmonary vein isolation (PVI) was achieved in more than 99 percent of patients in a first-in ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
Electrophysiology is one of the most rapidly-growing areas of healthcare. That may be why it’s the focus of so many ...
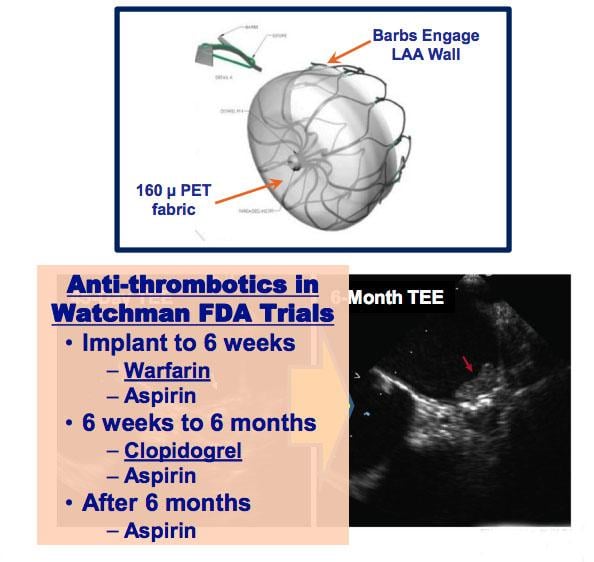
May 18, 2018 — Left atrial appendage closure (LAAC) with the transcatheter Watchman device prevents thromboembolism from ...
May 18, 2018 — Catheter ablation for atrial fibrillation (AF) in patients with heart failure in the CASTLE-AF Trial was ...


 June 20, 2018
June 20, 2018