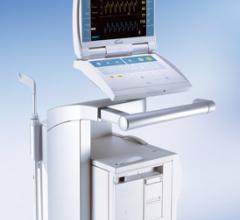
June 8, 2011 — GE Healthcare announced the Optima PET/CT 560, which offers efficiency and investment protection with the capability to grow with the future needs of a clinical practice.
June 8, 2011 – Research introduced at the Society of Nuclear Medicine's 58th annual meeting this week may lead to much-needed cardiovascular disease screening for diabetic patients at risk of ischemic heart disease. The disorder is marked by significantly reduced blood flow in the heart. Ischemia of the myocardium can signal diminished oxygenation of the heart tissue and trigger a heart attack if left untreated.
June 17, 2011 — The U.S. Food and Drug Administration (FDA) said Boston Scientific has issued a class I recall for its Innova Over-the-Wire Self-Expanding Stent System, because of complaints of no or partial deployments.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
June 17, 2011 — KLAS today announced the release of their highly anticipated "2011 Top 20 Best in KLAS Awards: Medical Equipment & Infrastructure" report. The awards are based on data from customer surveys of hospital and clinic executives, administrators, physicians, nurses, clinicians, and other directors and managers interacting with healthcare equipment and infrastructure solutions.
June 17, 2011 – In hybrid operating rooms (OR), healthcare facilities require a flexible imaging system to create a collaborative environment between clinicians and to allow both surgical and interventional procedures to be performed in one setting. At this year’s Society of Vascular Surgery (SVS) annual meeting, held in Chicago, June 16-18, Toshiba America Medical Systems, Inc. will highlight single-plane, ceiling-mounted Infinix-i X-ray systems and their compatibility with the Toshiba CAT-880B hybrid table and the Maquet Magnus OR table.
June 17, 2011 — Endologix, Inc. announced it received U.S. Food and Drug Administration (FDA) approval for AFX Endovascular AAA System for the treatment of abdominal aortic aneurysms (AAA). The company is introducing AFX at the annual meeting of the Society for Vascular Surgery (SVS), which is taking place June 16-18, 2011 in Chicago.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 17, 2011 – The new Pathfast cTnI-II (cardiac troponin I) test from Mitsubishi Chemical Medience Corporation has been granted 510(k) premarketing notification clearance by the U.S. Food and Drug Administration (FDA), clearing it for sale in the United States.
June 16, 2011 — More than 5,500 physicians, technologists, physicists, scientists and exhibitors gathered last week at the Society of Nuclear Medicine's (SNM) 2011 annual meeting, held June 4-8 in San Antonio, Texas.
Cordis announced it will cease production of the Cypher sirolimus-eluting stent, the first drug-eluting stent (DES) to secure U.S. Food and Drug Administration (FDA) approval, by the end of 2011. The company will also halt production of the Cypher Select Plus as well as development of the Nevo sirolimus DES.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Terumo Cardiovascular Systems Corp. has issued a class I recall for its Coronary Ostia Cannula 10 (25 cm) long. The company said foreign fragments of adhesive and plastic in the cannula tip may embolize, causing arterial injury, hemorrhaging or other serious events requiring unplanned surgery.
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Maquet Datascope Corp. has issued a class I recall for its System 98/98XT, CS100/CS100i and CS300 Intra-Aortic Balloon Pumps (IABPs) because of a defective fan in the power supply.
June 13, 2011 – Today, GE Healthcare Medical Diagnostics announced results of a study evaluating the cardiopulmonary safety of Optison (Perflutren Protein-Type A Microspheres Injectable Suspension, USP), a Food and Drug Administration (FDA)-approved diagnostic ultrasound contrast agent for use in improving suboptimal echocardiograms.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 14, 2011 – New performance measures for adults with coronary artery disease (CAD) and hypertension were released today by the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), and the American Medical Association (AMA)-convened Physician Consortium for Performance Improvement (PCPI). The measures reflect the standard of care for patients with coronary artery disease and hypertension, and are intended to provide practitioners and institutions with tools to measure and improve care quality.
June 14, 2011 — Lengthy periods of ambulance diversion are associated with higher mortality rates among patients with time-sensitive conditions, such as acute myocardial infarction. When a patient’s nearest emergency department was on diversion for 12 or more hours, there were higher patient mortality rates at 30 days, 90 days, nine months and one year than when not on diversion, according to a new study in the Journal of the American Medical Association.
The American Society of Echocardiography (ASE) and lifeIMAGE today announced a partnership that will give ASE members the ability to exchange medical images and data with anyone, anywhere.


 June 20, 2011
June 20, 2011