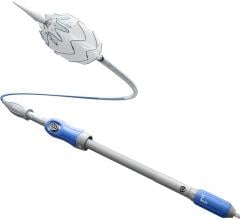
January 24, 2011 – A new device for treating peripheral artery disease (PAD) is now available in Europe. W.L. Gore and Associates announced the availability of its Viabahn Endoprosthesis on a lower profile delivery system at the Leipzig Interventional Course in Germany.
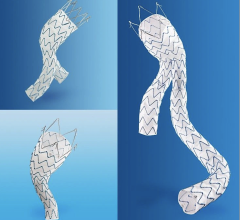
The device is designed to treat PAD percutaneously by relining the native vessel. The stent graft enables a reduction in delivery profile to 6 French for 5 and 6 mm devices and 7 French for 7 and 8 mm devices. It is delivered over a 0.18 inch or 0.14 inch guidewire. The reduced delivery profile will provide interventionalists with greater options for treating stenosis and occlusions of the superficial femoral artery (SFA) and other peripheral arteries where a small access size is critical.
It is the only device of its kind in Europe and is approved for endovascular grafting of peripheral arteries. The new delivery catheter is available in a 120 cm length and incorporates the Propaten Bioactive Surface, which utilizes end-point immobilization of derivatized heparin to the endoprosthesis luminal surface. This preserves the heparin bioactive sites such that they remain free to interact with the blood at the device surface without being consumed.
“A reduction in profile seen in the Gore Viabahn Endoprosthesis means easier endovascular delivery of the device to treat occlusions in the superficial femoral artery (SFA) and other tough to reach locations in patients where a small access size is critical and includes all of the historical benefits of heparin-bonded Propaten Bioactive Surface,” said Daniele Savio, M.D., vascular and interventional radiology, San Giovanni Bosco Hospital, Torino, Italy.
It is constructed with a durable, reinforced, biocompatible, ePTFE liner attached to an external nitinol stent structure. The excellent flexibility enables it to better traverse tortuous areas of the SFA and peripheral arteries and conforms to these arteries and withstands complex mechanical motion.
For more information: www.goremedical.com

 April 26, 2023
April 26, 2023