June 17, 2011 — Endologix, Inc. announced it received U.S. Food and Drug Administration (FDA) approval for AFX Endovascular AAA System for the treatment of abdominal aortic aneurysms (AAA). The company is introducing AFX at the annual meeting of the Society for Vascular Surgery (SVS), which is taking place June 16-18, 2011 in Chicago.
June 17, 2011 – The new Pathfast cTnI-II (cardiac troponin I) test from Mitsubishi Chemical Medience Corporation has been granted 510(k) premarketing notification clearance by the U.S. Food and Drug Administration (FDA), clearing it for sale in the United States.
June 16, 2011 — More than 5,500 physicians, technologists, physicists, scientists and exhibitors gathered last week at the Society of Nuclear Medicine's (SNM) 2011 annual meeting, held June 4-8 in San Antonio, Texas.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Cordis announced it will cease production of the Cypher sirolimus-eluting stent, the first drug-eluting stent (DES) to secure U.S. Food and Drug Administration (FDA) approval, by the end of 2011. The company will also halt production of the Cypher Select Plus as well as development of the Nevo sirolimus DES.
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Terumo Cardiovascular Systems Corp. has issued a class I recall for its Coronary Ostia Cannula 10 (25 cm) long. The company said foreign fragments of adhesive and plastic in the cannula tip may embolize, causing arterial injury, hemorrhaging or other serious events requiring unplanned surgery.
June 15, 2011 – The U.S. Food and Drug Administration (FDA) said Maquet Datascope Corp. has issued a class I recall for its System 98/98XT, CS100/CS100i and CS300 Intra-Aortic Balloon Pumps (IABPs) because of a defective fan in the power supply.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 13, 2011 – Today, GE Healthcare Medical Diagnostics announced results of a study evaluating the cardiopulmonary safety of Optison (Perflutren Protein-Type A Microspheres Injectable Suspension, USP), a Food and Drug Administration (FDA)-approved diagnostic ultrasound contrast agent for use in improving suboptimal echocardiograms.
June 14, 2011 – New performance measures for adults with coronary artery disease (CAD) and hypertension were released today by the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), and the American Medical Association (AMA)-convened Physician Consortium for Performance Improvement (PCPI). The measures reflect the standard of care for patients with coronary artery disease and hypertension, and are intended to provide practitioners and institutions with tools to measure and improve care quality.
June 14, 2011 — Lengthy periods of ambulance diversion are associated with higher mortality rates among patients with time-sensitive conditions, such as acute myocardial infarction. When a patient’s nearest emergency department was on diversion for 12 or more hours, there were higher patient mortality rates at 30 days, 90 days, nine months and one year than when not on diversion, according to a new study in the Journal of the American Medical Association.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The American Society of Echocardiography (ASE) and lifeIMAGE today announced a partnership that will give ASE members the ability to exchange medical images and data with anyone, anywhere.
June 14, 2011 – The first successful Chinese implants of the Edwards SAPIEN XT valve were performed in May.
June 14, 2011 – An initial animal study of a next-generation transcutaneous C-Pulse Heart Assist System has been completed. The system is made by Sunshine Heart Inc., a global medical device company focused on technologies for Class III/ambulatory Class IV (moderate to severe) heart failure.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
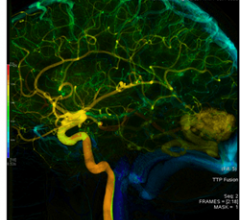
June 14, 2011 –FDA has given clearance to AngioViz, a GE application that gives doctors a new visualization of vascular flow on a single image to help them make important decisions during complex interventional radiology procedures.
Expanding upon its radiopharmacy platform, GE Healthcare is introducing new tracers to the FASTlab multi-tracer platform, an advanced positron emission tomography (PET) chemistry system on which the company is also developing PET proprietary agents. The system offers a number of significant enhancements to address the ever-evolving challenges of tracer production. With the addition of three new cassettes, users can access and produce FDG phosphate formulation, NaF, FMISO (HPLC free), FLT (HPLC free) and FDG citrate formulation (equivalent to TRACERlab MX standard formulation) on the same platform. For more information: www.gehealthcare.com
Research being presented at Society of Nuclear Medicine’s (SNM) 58th annual meeting shows that molecular imaging is helping to identify and treat a silent killer. A study focusing on hypertrophic cardiomyopathy (HCM) — a cardiovascular disorder that causes a thickening of the heart muscle — is proving that the effects of a genetic mutation may be an important key to understanding the disease. In related research, a treatment called alcohol septal ablation is being revealed as an effective treatment for severe cases of HCM.


 June 17, 2011
June 17, 2011