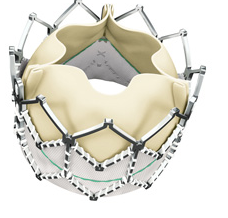
June 14, 2011 – The first successful Chinese implants of the Edwards SAPIEN XT valve were performed in May.
The transfemoral valve implantations were performed as special access cases at the Second Military Medical University, under a joint educational and training program on transcatheter aortic valve implantation between the university and Edwards Lifesciences.
"Patients in China are in need of a transcatheter tissue valve treatment option, and we hope to gain the necessary approvals to launch the Edwards SAPIEN XT valve in China as early as 2013," said Huimin Wang, M.D., Edwards' corporate vice president, Japan, Asia Pacific and Latin America.
The Edwards SAPIEN XT valve was approved for sale in Europe in March 2010. It is an investigational device in the United States, Japan and China.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









