February 15, 2012 — Vessix Vascular Inc., developer of a novel percutaneous radiofrequency (RF) balloon catheter technology, announced that it will make its first detailed public presentation of preclinical data relating to its new V2 Renal Denervation System on Saturday, Feb. 18, 2012 at the TRenD Workshop 2012, in Frankfurt, Germany.
February 15, 2012 — CardioNet Inc. a wireless medical technology company announced the acquisition of ECG Scanning and Medical Services Inc.
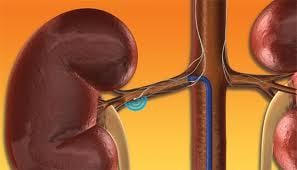
February 15, 2012 — Medtronic Inc. announced the start of two clinical initiatives evaluating the broader, real-world clinical use of the company’s Symplicity renal denervation system across multiple conditions.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 14, 2012 — The American College of Radiology (ACR) called on Congress to reject what would be the eighth cut to Medicare funding for imaging scans in the last six years and protect the ability of seniors to receive this live-saving care.
February 10, 2012 — Researchers from the National Institute of Advanced Industrial Science and Technology (AIST), Japan, determined arterial stiffness using the relationship between cuff pressure and arterial volume.
February 10, 2012—Merit Medical Systems recently announced it acquired the assets of Ostial Solutions LLC, maker of the Ostial Pro stent positioning system.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
February 9, 2012 — The U.S. Food and Drug Administration (FDA) sent a warning letter to Merit Medical Systems Inc. regarding modifications to the hydrophilic coating process for the Merit Laureate Hydrophilic Guidewire, following an inspection of the company's facility in Galway, Ireland.
February 9, 2012 — Stereotaxis Inc. said its Vdrive robotic navigation system, which provides physicians the ability to remotely manipulate traditionally non-robotic catheters, is growing in popularity and is expected to surpass 500 clinical procedures in Europe in February.
February 9, 2012 — Boston Scientific announced the United States launch of the TruePath CTO device, designed to facilitate the crossing of chronic total occlusions (CTOs, or complete blockages) within the peripheral vasculature.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
St. Jude Medical has launched its Unify Quadra cardiac resynchronization therapy defibrillator (CRT-D) and Quartet left ventricular quadripolar pacing lead. It features four electrodes spaced over 4.7 cm, enabling up to 10 pacing configurations for better cardiac rhythm management.
February 9, 2012 — Healthcare imaging specialist Barco announced that it has signed an agreement to acquire U.K.-based JAOtech, a manufacturer of patient entertainment and point-of-care terminals for hospitals.
February 9, 2012 — Valley Presbyterian Hospital (VPH) was the site for the first implantation in the United States of a new heart device since the U.S. Food and Drug Administration (FDA) approved the DF4 High-Voltage Connector System by Medtronic earlier this month, Gus Valdespino, VPH president and CEO announced.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 9, 2012 — DSM announced a successful collaboration with medical manufacturer EPflex Feinwerktechnik GmbH to launch a new set of guidewires with ComfortCoat hydrophilic coating, a proprietary DSM technology.
February 9, 2012 — Wolters Kluwer Health announced King’s Daughters Medical Center has selected its ProVation MD software for cardiology procedure documentation and coding in its cardiac catheterization labs.
A 3-D view does more than make for exciting movies in a theater; it can also improve the care of animals. The Chicago Zoological Society’s (CZS) Brookfield Zoo is the first North American zoo to use revolutionary 3-D imaging technology with on-site digital radiology and computed tomography (CT) equipment in its state-of-the-art animal hospital. The new technology allows the Society’s veterinarians to enhance two-dimensional CT scans, MRIs and ultrasounds with 3-D models that will enable them to better treat zoo animals.


 February 15, 2012
February 15, 2012











