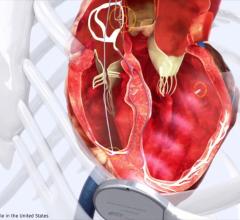
January 26, 2012 — St. Jude Medical recently announced U.S. Food and Drug Administration (FDA) approval of its Unify Quadra cardiac resynchronization therapy defibrillator (CRT-D) and Quartet left ventricular quadripolar pacing lead.
The Unify Quadra CRT-D and Quartet lead offer physicians the ability to effectively and efficiently manage the ever-changing needs of patients with heart failure. The system integrates multiple pacing configurations and tailored therapy features that enable physicians to optimize the system at implant and follow-up, as well as better manage common pacing complications without having to surgically reposition the lead. The system is also approved for remote patient management utilizing the Merlin.net Patient Care Network.
The Quartet lead – used as part of the Unify Quadra system – features four electrodes spaced over 4.7 cm, enabling up to 10 pacing configurations. Multiple pacing configurations allow the physician to implant the lead in the most stable position without making trade-offs in electrical performance. This includes pacing closer to the base of the left ventricle, which recent studies associate with better patient outcomes, and which may be more difficult with traditional bipolar leads. The quadripolar pacing electrodes also provide physicians more options to optimize CRT performance, such as pacing around scar tissue in the heart and avoiding the most common pacing complications. The many benefits conferred from the Quartet lead’s unconventional pacing have been demonstrated by implanters around the world and reported in a number of published studies.
Common pacing complications that can occur in patients implanted with a CRT system include high pacing thresholds and unintentional phrenic nerve or diaphragmatic stimulation. Patients with high pacing thresholds require significantly higher energy to pace the heart; this may reduce the device's battery life requiring patients to have more surgeries to replace devices or cause pacing to be ineffective. Phrenic nerve and diaphragmatic stimulation occur when the electrical output from a device inadvertently activates the diaphragm muscle (either directly or via the phrenic nerve), causing patients to hiccup with the delivery of the pacing stimuli. In particular, phrenic nerve and diaphragmatic stimulation may be body-position sensitive and not evident at the time of the implantation procedure, while the patient is lying on their back. Both high pacing thresholds and phrenic nerve or diaphragmatic stimulation are often due to the location of the pacing lead electrode and with limited pacing options, may require that the lead be repositioned surgically or CRT be disabled.
CRT, which can be delivered by an implantable cardioverter defibrillator (ICD), resynchronizes the beating of the heart's lower chambers (ventricles), which often beat out of sync in heart failure patients. Studies have shown that CRT can improve the quality of life for many patients with heart failure, a progressive condition in which the heart weakens and loses its ability to pump an adequate supply of blood. Approximately 23 million people worldwide are afflicted with congestive heart failure (CHF), and 2 million new cases of CHF are diagnosed each year worldwide.
For more information: www.sjmquadripolar.com


 July 21, 2025
July 21, 2025