
As vendors develop, and hospitals shop for, electrophysiology (EP) information and reporting systems, there are several considerations that will contribute to the efficiency and longevity of these systems.
August 29, 2012 — A new, daytime teleradiology company targeting urgent care, independent diagnostic testing facilities (IDTFs), mobile medical and physician offices is now serving clients in 10 states, is licensed in 20 and plans to expand with the growth of its customers. Teleradiology Specialists keeps overhead costs low and extends the savings to every customer while employing highly qualified radiologists who are available to read a wide variety of studies all day.
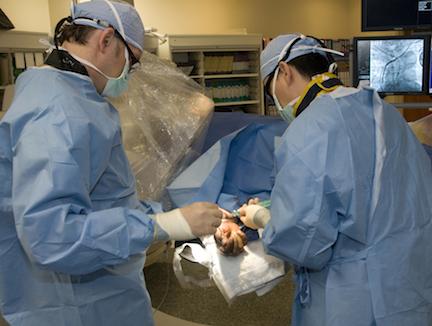
Recently, concerns have been raised that transradial (TR) cardiac catheterization significantly increases radiation exposure when compared with the conventional transfemoral (TF) approach. The current rapid uptake of TR in the United States is mainly due to its economic benefits and overall favorable patient outcomes. Perhaps most importantly, TR provides a measurable reduction in vascular complications, which translates to shorter time for ambulation and post-procedural stay. The reduction in bleeding with TR likely lowers mortality in patients with ST-elevation myocardial infarction (STEMI). One potential reason for the slow adoption of TR may be the perceived concern about increased radiation exposure.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Patients with 2C19 that is less active due to loss of gene copies are poor metabolizers of clopidogrel, opening patients to the possibility of a higher rate of mayocardial infacrtion (MI). There are assays for platelet function response (VerifyNow) and genetic tests to measure patient response, which could help determine if generic clopdogrel or more expensive agents should be used.

The standard-of-care for dual antiplatelet therapy (DAPT) for more than a decade has been clopidogrel (Plavix) plus aspirin. This standard is now being challenged by new agents — ticagrelor (Brilinta) and prasugrel (Effient). While the new agents are more effective at preventing platelet aggregation than clopidogrel, each has unique characteristics. Plavix went generic earlier this year, which may also impact decisions about which therapies to use.
August 28, 2012 — Toshiba America Medical Systems Inc. has received U.S. Food and Drug Administration (FDA) clearance on its new Vantage Titan 1.5T series, which features the 8-, 16- and 32-channel MR (magnetic resonance) systems.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
August 28, 2012 — The International Contrast Ultrasound Society (ICUS) applauded the U.S. Food and Drug Administration’s (FDA) decision to modify the U.S. product label for several ultrasound contrast agents used to improve the accuracy of radiation-free ultrasound scans.
August 28, 2012 — GE Healthcare announced important changes to the U.S. product label for Optison (perflutren protein-type A microspheres injectable suspension, USP), a contrast agent that may improve visualization of the left ventricular border, an area of the heart that is critical to see in order to diagnose certain heart diseases such as hypertrophic cardiomyopathy.
August 28, 2012 — NDS Surgical Imaging (NDSsi) is now shipping its new Dome GX4MP radiology display, a 30-inch widescreen model offering multimodality viewing in both color and grayscale.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

August 27, 2012 — A recent multi-center study, led by researchers from Wake Forest School of Medicine and published in the Journal of the American Medical Association (JAMA) has indicated that computed tomography (CT) scanning of the heart and measuring the coronary artery calcium (CAC) score is the most accurate predictor of cardiovascular disease for individuals at intermediate risk for heart disease.

August 27, 2012 — Positron Corp., a molecular imaging healthcare company, announced the submission of a drug master file (DMF) with the U.S. Food and Drug Administration (FDA) for the production of active pharmaceutical ingredient- (API) grade strontium-82 through its wholly owned subsidiary, Manhattan Isotope Technology LLC (MIT).

There are some key requirements specifically aimed at imaging and radiology included in the Stage 2 meaningful use requirements released Aug. 23 by the Department of Health and Human Services (HHS). HHS fielded many concerns and questions about these requirements and offered answers in the 642-page document.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

August 24, 2012 — The Stage 2 meaningful use requirements for electronic health records (EHRs) were released Aug. 23 by the Department of Health and Human Services (HHS) and the Centers for Medicare and Medicaid (CMS). The rule specifies criteria that eligible professionals (EPs), eligible hospitals and critical access hospitals (CAHs) must meet in order to qualify for CMS EHR incentive payments. In addition, it specifies payment reductions for EPs and facilities failing to demonstrate meaningful use of certified EHR technology.
August 23, 2012 — The U.S. Food and Drug Administration (FDA) has cleared Toshiba’s Aquilion RXL Edition CT system. The system reconstructs images faster and includes the latest dose reduction technologies, providing faster, safer information to physicians and patients.
August 23, 2012 — Decision Resources, a research and advisory firm for pharmaceutical and healthcare issues, finds that the 12-month post-hospital acute coronary syndrome market will initially contract from just under $2 billion in 2011 to about $1.6 billion in 2013, mainly as a result of the U.S. genericization of clopidogrel (Bristol-Myers Squibb/Sanofi’s Plavix) in 2012. Decision Resources forecasts that the post-hospital acute coronary syndrome antiplatelet/anticoagulant market will then expand to almost $2.7 billion in 2021, owing largely to uptake of oral agents with novel modes of action, such as AstraZeneca’s ticagrelor (Brilinta) and the CETP inhibitors, Merck’s anacetrapib and Eli Lilly’s evacetrapib.

 August 29, 2012
August 29, 2012







