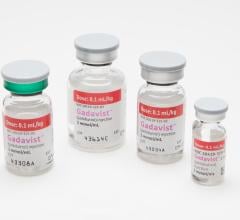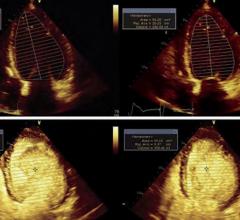
August 28, 2012 — GE Healthcare announced important changes to the U.S. product label for Optison (perflutren protein-type A microspheres injectable suspension, USP), a contrast agent that may improve visualization of the left ventricular border, an area of the heart that is critical to see in order to diagnose certain heart diseases such as hypertrophic cardiomyopathy. Optison is indicated for use in patients with suboptimal echocardiograms to opacify the left ventricle and to improve delineation of the left ventricular endocardial borders.
Optison is not for use in patients with known or suspected: (1) Right-to-left, bi-directional or transient right-to-left cardiac shunts, or (2) hypersensitivity to perflutren, blood, blood products or albumin. It should not be administered by intra-arterial injection.
The agent carried a U.S. Food and Drug Administration (FDA) boxed warning, stating serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following perflutren-containing microsphere administration. Most serious reactions occur within 30 minutes of administration.
After a review of GE Healthcare’s March 2012 supplemental new drug application for proposed label changes, the FDA made revisions to the prescribing information for Optison including:
- Removal of the following statement from within the previous boxed warning: “In patients with pulmonary hypertension or unstable cardiopulmonary conditions, monitor vital sign measurements, electrocardiography and cutaneous oxygen saturation during and for at least 30 minutes after Optison administration.” Similar language was also removed from the warning section of the label.
- Addition of the following statement to the boxed warning: “Most serious reactions occur within 30 minutes of administration,” which is consistent with current information included in the warning section.
- Addition to the clinical trails section, describing the results of the Optison pulmonary hemodynamic study.
- Addition of the further qualifier in the following statement in the warning section: “Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following perflutren-containing microsphere administration, typically within 30 minutes of administration.”
“GE Healthcare is committed to providing safe, innovative and effective medical products that aid in the detection of cardiovascular diseases, and we are pleased that the FDA label change supports this goal,” said Mark Gelder, global head of medical affairs and clinical development at GE Healthcare Medical Diagnostics. “The approved Optison labeling revisions are based on data from clinical and surveillance studies and more than 12 years of post-marketing clinical experience which found no statistically significant risks or safety signals. The revised label may increase access of Optison in critically ill patients who may receive the most benefit from contrast-enhanced echocardiography.”
The most frequently reported adverse reactions following clinical trial use of Optison were headache, nausea and/or vomiting, warm sensation or flushing, and dizziness. Cardiac arrests and other serious, but non-fatal, adverse reactions were uncommonly reported post-marketing. Most of these uncommon reactions included cardiopulmonary symptoms and signs such as cardiac or respiratory arrest, hypotension, supraventricular and ventricular arrhythmias, respiratory distress or decreased oxygenation. Reports also identified neurologic reactions (loss of consciousness or convulsions) as well as anaphylactoid reactions.
Optison remains an important diagnostic option for patients with suboptimal echocardiograms.
Additionally, Optison is stable at room temperature for up to 24 hours and takes less than 60 seconds to prepare, allowing for quick access to contrast in the lab, trauma situations or during transport from one hospital campus to another. Optison is for single use only. Users should follow labeled instructions for product handling, and use and discard unused product properly.
"The revised product labeling for Optison better reflects the known safety profile of this agent and should encourage ultrasound contrast agent use in patients most likely to realize the greatest incremental diagnostic benefit—hospitalized patients with critical illnesses, including those with known significant cardiopulmonary disease,” said Michael L. Main, M.D., medical director, cardiovascular ultrasound imaging laboratory, St. Luke’s Mid-America Heart Institute, Kansas City, Mo.
For more information: www.optisonimaging.com


 August 17, 2023
August 17, 2023