Endologix Inc. announced that it has received Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin a clinical trial to evaluate the safety and effectiveness of the Nellix EndoVascular Aneurysm Sealing System (EVAS).

St. Jude Medical Inc. received European CE mark approval for its 25-mm Portico Transcatheter Aortic Heart Valve Implantation System.
Medtronic Inc. announced that the first patients were randomized in Symplicity HTN-4, which will evaluate the Symplicity renal denervation system in patients with moderate uncontrolled hypertension.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
An investigational, manmade blood vessel used in vascular grafts for kidney dialysis patients may potentially show encouraging early results among study patients in Poland, according to preliminary data reported by a researcher at Duke Medicine.
Massachusetts was on the same brink in 2006 that the entire nation is on today: the brink of expanding health insurance to cover far more people than before through government-driven, market-based reform.
A study published in the Journal of the American Heart Association revealed findings that may impact diagnostic strategies and clinical decision-making for patients with suspected coronary artery disease (CAD).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
To the gratification of the Healthcare Information and Management Systems Society (HIMSS), the U.S. Department of Health and Human Services, the Centers for Medicare and Medicaid Services and the Office of the National Coordinator for Health IT have heard concerns from health stakeholders and extended Meaningful Use Stage 2 by one year.
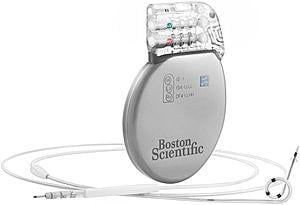
Boston Scientific Corp. has received CE mark approval of its X4 line of quadripolar cardiac resynchronization therapy defibrillator (CRT-D) systems, including the AutoGen X4, DynaGen X4 and InoGen X4 CRT-Ds, a suite of Acuity X4 quadripolar LV leads and the Acuity Pro lead delivery system.
Digisonics and Medis have partnered to provide a single system solution for image analysis and structured reporting of pediatric and adult cardiovascular magnetic resonance (MR) studies.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A University of Alabama at Birmingham surgical team has performed the first surgery using a virtual augmented reality technology called VIPAAR (Virtual Interactive Presence in Augmented Reality) in conjunction with Google Glass, a wearable computer with an optical head-mounted display.

Comprised of the cardiologist and the heart surgeon, the heart team collaboratively assesses and determines the best course of treatment for cardiac patients. ECHI has a large and successful surgical and percutaneous valve program built on the heart team concept. This approach is also used for treating patients with coronary artery disease by applying the use of fractional flow reserve (FFR).
A report released by IMV Medical Information Division found that providers of nuclear medicine (NM) services are consolidating as procedure volume dropped in the subspecialty from 2008 to 2012.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The National Institutes of Health announced that a network of 25 regional stroke centers working with nearby satellite facilities will span the country, have teams of researchers representing every medical specialty needed for stroke care and will address the three prongs of stroke research: prevention, treatment and recovery.
AtriCure Inc., an atrial fibrillation (AF) medical device provider, and Endoscopic Technologies Inc, doing business as “Estech,” announced that they have entered into a definitive merger agreement under which AtriCure has agreed to acquire Estech.

A study shows that coronary artery calcium (CAC) screening, an assessment tool that is not currently recommended for people considered at low risk, should play a more prominent role in helping determine a person’s risk for heart attack and heart disease-related death, as well as the need for angioplasty or bypass surgery.

 January 03, 2014
January 03, 2014