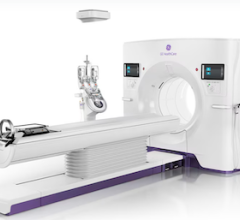
August 13, 2013 — IMRIS Inc. has obtained regulatory CE mark for Visius iCT, the first and only ceiling-mounted intraoperative computed tomography (CT), allowing for sales and marketing in the European Union.
IMRIS received U.S. Food and Drug Administration (FDA) 510(k) clearance for Visius iCT on July 18, 2013. The company now has systems sold to three major eastern U.S. neurosciences centers with installations in progress at two of these centers.
Visius iCT provides personalized radiation dose management together with diagnostic quality imaging during surgical procedures to assist surgeons in critical decision making and procedure guidance. The 64-slice scanner effortlessly moves into and out of the operating room (OR) in about 30 seconds during surgery using ceiling-mounted rails to ease workflow. The scanner can travel into two adjacent ORs to allow the hospital to use it for more patients.
Patient transport and need for floor-mounted rails used by other systems are eliminated which opens up valuable OR space and allows unimpeded movement of surgical equipment and maintaining familiar surgical protocols, workflow and infection control procedures. The Visius iCT also offers the longest scanner travel range on the market today.
Minimizing radiation dose is critical. VISIUS iCT features a suite of software applications such as 3-D volume rendering to aid in surgical planning and dose reduction algorithms which consider a patient's unique characteristics and imaging target to maximize image quality and minimize dose. The system software allows dosage visualization prior to the scan and adjustments based on specific clinical need with detailed dosage reports produced after each scan.
For more information: www.imris.com


 February 02, 2026
February 02, 2026