October 26, 2023 — HeartFlow, Inc., a leader in non-invasive integrated artificial intelligence (AI) heart care solutions, today announced results from three clinical studies supporting the use of advanced coronary computed tomography angiography (CCTA)-based technologies in the assessment of patients with suspected coronary artery disease (CAD). Results from the ADVANCE-DK Plaque, EMERALD II, and FASTTRACK-CABG studies were presented at the Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco.
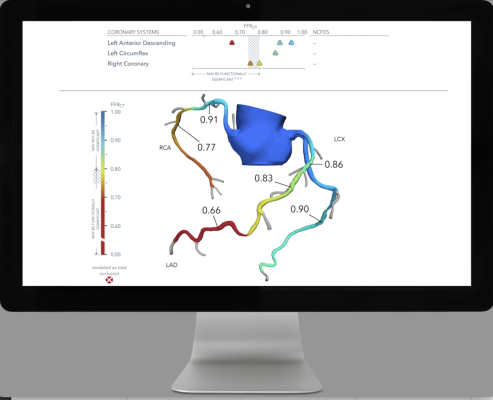
The data build on HeartFlow’s extensive body of clinical evidence supporting AI-powered non-invasive diagnostic testing for patients with CAD. HeartFlow is the first company to offer a suite of technologies to help clinicians non-invasively identify and quantify narrowings in the coronary arteries (RoadMapAnalysis), assess the impact of narrowings on coronary blood flow (FFRCT Analysis), and characterize and quantify coronary atherosclerosis (Plaque Analysis). Together, this comprehensive solution offers valuable data not currently available with traditional non-invasive diagnostics tools.
ADVANCE-DK Plaque, the Danish subset from the larger ADVANCE Registry, evaluated utility and clinical outcomes of HeartFlow’s AI-enabled FFRCT Analysis and Plaque Analysis in clinically stable, symptomatic patients with CAD. Three-year data confirm that patients with both abnormal FFRCT and high plaque burden had 2.5 - 3 times higher risk of adverse cardiac events than patients with abnormalities of only FFRCT or plaque. HeartFlow’s FFRCT and Plaque analyses provide a robust assessment of prognosis, allowing physicians to better understand CAD risk borne by their patients.
EMERALD II, a multinational, multicenter study, assessed the cardiovascular function and plaque features of patients with acute coronary syndrome (ACS) leveraging comprehensive CCTA analysis, including per-lesion and per-vessel plaque quantification and hemodynamic analysis. Building from a prior study, EMERALD, data further clarify that lesion-specific physiology has high prognostic value, and in combination with HeartFlow's Plaque Analysis, can help identify patients at increased risk of adverse cardiac events. Using a best-fit model combining anatomy, lesion-specific physiology, and quantified coronary plaque characteristics, the investigators were able to predict lesions that progressed to cause ACS events with much greater discrimination than traditional CCTA tools.
“EMERALD II demonstrates that AI-enabled lesion-specific physiology data combined with quantitative coronary plaque assessment is powerfully prognostic in identifying high-risk coronary artery disease that may result in a heart attack,” said Professor Bon-Kwon Koo, Director of Cardiovascular Center and Chair in Cardiology Division at Seoul National University Hospital. “These combined tools provide actionable insight that will help us improve outcomes in our patients.”
FASTTRACK-CABG is a single-arm, multicenter, prospective study assessing safety and feasibility of planning and execution of bypass surgery in patients with complex CAD, solely based on CCTA combined with FFRCT. FASTTRACK-CABG is an investigator-driven study sponsored by the University of Galway, Ireland and co-funded by HeartFlow and GE HealthCare. Data demonstrate the ongoing expansion for a CCTA-driven pathway and support the use of non-invasive diagnostics for patients of varying CAD complexities. In 99% of high disease-burden patients referred for coronary artery bypass grafting (CABG), treatment planning with CCTA and FFRCT was feasible as an alternative to Invasive coronary angiography (ICA). Importantly, this alternative approach to CABG planning was found to be safe.
“FASTTRACK-CABG is further evidence of CCTA and FFRCT’s role as the emerging standard of care for planning the treatment of patients with increasing levels of cardiovascular disease, above and beyond this pathway’s already established role in disease diagnosis,” said Professor Patrick Serruys, MD, Ph.D., eFESC, FACC, Professor of Interventional Medicine and Innovation at University of Galway, Ireland. “Patients will benefit from avoidance of unnecessary invasive assessment as surgeons, interventionalists, and imagers embrace CCTA and FFRCT.”
“We are immensely grateful to the participants and investigators in these landmark studies,” said Campbell Rogers, Chief Medical Officer, HeartFlow. “We are also pleased to continue providing robust clinical evidence as to how our products can help health care providers manage people with coronary artery disease.”
For more information: www.heartflow.com


 October 31, 2025
October 31, 2025 









