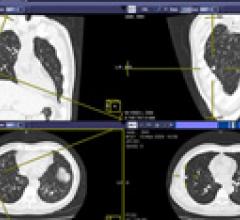
At RSNA 2013, GE Healthcare will showcase a range of molecular imaging tools designed to enable treatment evaluation earlier in a patient’s care pathway. Q.Suite, a collection of capabilities designed to extend quantitative positron emission tomography (PET) by generating more consistent standardized uptake value (SUV) readings, enabling clinicians to assess treatment response. Q.Suite is designed to help improve consistency of quantitative measurements in every key area: daily quality control, scanner workflow, motion correction, reconstruction algorithms and analysis and reporting applications. Controlling these variables to increase consistency can improve the clinician’s confidence that an SUV change has useful clinical meaning.
When echocardiograms prove insufficient for diagnosis, Diagnosoft’s offers a patented and U.S. Food and Drug Administration 510(k)-cleared cardiac magnetic resonance imaging (CMR) solution for patient assessment. With benchmark sensitivity and specificity, the high throughput Diagnosoft CMR exam yields an advantageous alternative to nuclear or cardiac cath exams.
Independent global research organization Frost & Sullivan honored Barco, a healthcare imaging solutions company, with the 2013 North American Product Line Strategy Award in Diagnostic Imaging Displays at the 2013 Growth, Innovation & Leadership Awards on Sept. 9, 2013 in San Jose, Calif. The award recognized Barco for delivering excellence and best practices in the North American diagnostic image displays market.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The ScottCare Corp. announced Innovo, a rechargeable digital transmitter for the company’s VersaCare multi-parameter telemetry solution serving cardiopulmonary rehabilitation clinics throughout the U.S. and internationally. ScottCare will feature the Innovo unit at this year’s Annual Meeting of the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) October 3-5 in Nashville, Tenn.
Fabry disease can be detected using a new magnetic resonance imaging (MRI) technique that was developed at the University of Alberta. The discovery of this new diagnostic tool has resulted in updated clinical guidelines for the diagnosis and treatment of Fabry disease in Canada.
While 95 percent of cardiac catheterizations in the United States are done through the groin, physicians at Bethesda Heart Hospital are utilizing special training to offer patients a safer, more comfortable procedure.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
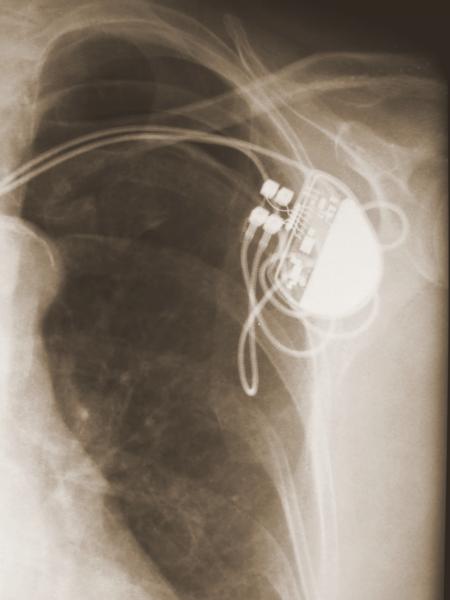
Devices like pacemakers and defibrillators allow doctors to introduce electrical signals to set patients' hearts at regularly timed beats. However, these small mechanical devices come with risks.
Infinitt will introduce its new “Communicator” package at RSNA 2013. It is an add-on that ensures immediate communication of critical test results, provides peer review and emergency room discrepancy management and includes an embedded communication feature that allows real-time collaboration between users of the Infinitt picture archive and communications system (PACS). This is particularly valuable for clarification between the study’s dictating physician and transcriptionist or the referring physician.

Early treatment of heart attack patients with an inexpensive beta-blocker drug called metoprolol, while in transit to the hospital, can significantly reduce damage to the heart during a myocardial infarction, according to clinical trial/study results published Oct. 1 in the journal Circulation. The study was a collaboration between Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) and Icahn School of Medicine at Mount Sinai.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

As a cutting-edge technique, many hospitals around the country are investigating the development of their own TAVR programs. Starting a TAVR program requires a collaborative commitment to care and advanced imaging technology to plan and perform the exams. With the right approach and equipment, a TAVR program can have a profound impact on patients and the hospitals that serve them.
Sudden cardiac death (SCD) is the leading cause of death in high school-aged athletes. To determine if SCD can be prevented with a heart screening, The Christ Hospital Health Network (Cincinnati, Ohio), The University of Mississippi Medical Center and Cincinnati Children’s Hospital Medical Center partnered with USRowing and Toshiba America Medical Systems Inc. to conduct The Athlete Heart Research Study. Initial participants included volunteer high school rowers with more than two years of continuous practice who competed in the USRowing Youth National Championships, June 7 – 9, 2013, in Oak Ridge, Tenn.

The U.S. Food and Drug Administration (FDA) released a draft of its proposed updates for the non-clinical engineering tests and recommended labeling for intravascular stents and associated delivery systems. It is designed to provide draft guidance for industry and FDA staff.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Methodist Le Bonheur Healthcare (MLH) will deploy McKesson’s portfolio of enterprise medical imaging products across its facilities to improve physician collaboration, streamline data management and simplify its imaging archive process with a single point of access.
Rochelle Community Hospital purchased and installed Carestream’s Vue Cardio PACS (picture archive and communications system) that provides fully featured viewing and management for its echo cardiograms and nuclear cardiology exams. This versatile system will also support ECGs and other types of cardiac exams that the critical access hospital may add in the future.
The U.S. Food and Drug Administration (FDA) approved Biotronik’s Ilesto family of implantable cardioverter-defibrillator/cardiac resynchronization therapy defibrillators (ICD/CRT-D devices). Biotronik follows this with the launch of its next generation technology platform Ilesto DX.

 October 03, 2013
October 03, 2013