Digisonics (Booth #211) will showcase its Enterprise PACS and Structured Reporting Solutions for all imaging study types at this year’s Society for Imaging Informatics in Medicine (SIIM) Annual Meeting in Long Beach, Calif.
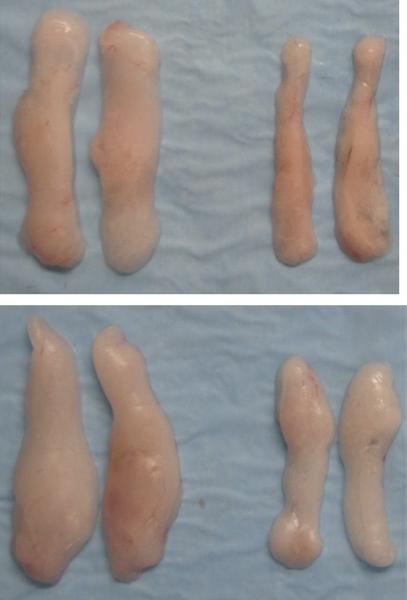
A study in The Journal of Experimental Medicine shows that I?B kinase ? (IKK?) functions in smooth muscle cells to regulate vascular inflammatory responses and atherosclerosis development.
CathMaps demonstrated how mobile technology can provide emergency assistance and peace of mind to millions of Americans at risk for a cardiac event.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
OrbusNeich announced the launch of the Sapphire II NC coronary dilatation catheter, a non-compliant balloon catheter engineered to cross tight lesions, outside the United States.
The National Lipid Assn. (NLA) released a draft summary that highlights key aspects of care that provide a thorough supplement and direction for clinicians treating patients with dyslipidemia.
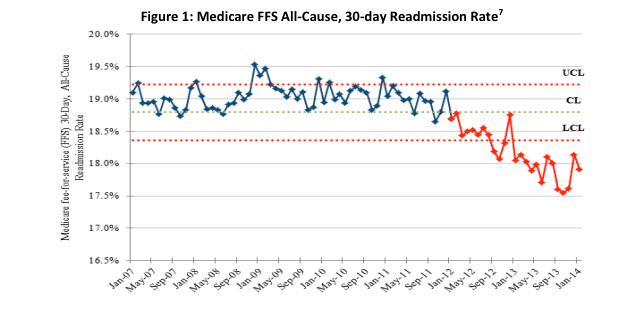
May 14, 2014 — The Department of Health and Human Services (HHS) announced that new preliminary data show an overall 9 percent decrease in hospital-acquired conditions nationally during 2011 and 2012. National reductions in adverse drug events, falls, infections and other forms of hospital-induced harm are estimated to have prevented nearly 15,000 deaths in hospitals, avoided 560,000 patient injuries and approximately $4 billion in health spending over the same period.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

May 14, 2014 — Data presented during Heart Rhythm 2014 found the use of quadripolar leads reduced the number of hospitalizations by 53 percent when compared to the non-quadripolar group. This hospitalization rate reduction translated into a statistically significant 62 percent reduction in overall costs for both healthcare systems and patients.

May 14, 2014 — Vivek Reddy, M.D., director of arrhythmia services for The Mount Sinai Hospital, reported promising 12-month follow-up data showing the world's first leadless pacemaker is demonstrating overall device performance comparable to conventional pacemakers. Reddy presented the one-year LEADLESS study data findings during a late-breaking clinical trial presentation on Heart Rhythm 2014, the Heart Rhythm Society's (HRS) 35th annual scientific sessions in San Francisco.
May 14, 2014 — Detroit Medical Center (DMC) Harper Hospital presented a live CorPath robotic-assisted percutaneous coronary intervention (PCI), or coronary angioplasty, during the American College of Cardiology (ACC) annual meeting this year. The procedure, which was transmitted live to Washington, D.C., for meeting attendees, was led by Ted Schreiber, M.D., president of the Cardiovascular Institute and director of the Cardio One team at DMC Harper Hospital.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

May 13, 2014 — The Heart Rhythm Society (HRS), American College of Cardiology (ACC) and American Heart Association (AHA) released Expert Consensus Statement on the Use of Implantable Cardioverter Defibrillator Therapy in Patients Who Are Not Included or Not Well Represented in Clinical Trials at Heart Rhythm 2014, the 35th annual scientific sessions of HRS. The expert consensus statement provides first-of-its-kind guidance on ICD therapy for the management of patient populations who are not well represented in clinical trials and, as a result, not specifically included in existing guidelines.
Esaote North America received U.S. Food and Drug Administration (FDA) clearance to market and sell its Virtual Navigator fusion imaging technology for ultrasound to U.S. customers.

A Boston Scientific Corp. study demonstrated that outcomes for patients with the company's transvenous implantable cardioverter defibrillators (ICD) who received routine defibrillation testing (DT) at implant were similar to outcomes for those patients who did not receive defibrillation testing.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Elixir Medical Corp. received CE mark approval for its DESolve 100 Novolimus Eluting Bioresorbable Coronary Scaffold System.
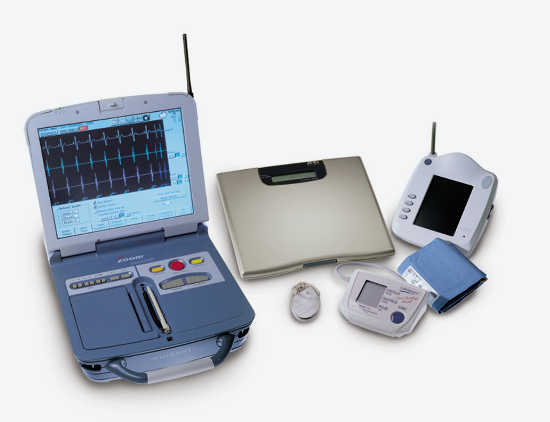
May 12, 2014 — Patients using the Boston Scientific Latitude remote patient management system with wireless telemetry demonstrated significantly lower mortality and fewer hospitalizations than patients with Latitude-compatible devices who were not followed on the system, according to results from the PREDICt-RM study (Patient RElated Determinants of ICD Remote Monitoring Utilization and Outcomes). The results were presented at Heart Rhythm 2014, the Heart Rhythm Society's (HRS) 35th annual scientific sessions in San Francisco.
May 12, 2014 — The Center for Magnetic Resonance Research (CMRR) at the University of Minnesota, Minneapolis, is the first U.S. facility to install Siemens Healthcare’s Magnetom Prisma, a 3T magnetic resonance imaging (MRI) scanner capable of exceptionally high spatial and temporal resolution to achieve image quality, particularly in highly demanding situations.

 May 15, 2014
May 15, 2014








