May 27, 2014 — New research published in Nature’s Scientific Reports identifies a new type of light sensor that could allow medical and security imaging via low-cost cameras.
The U.S. Food and Drug Administration (FDA)-cleared EKG Glove by IneedMD Inc., based in New York, has been recognized as one of the most cutting edge medical technologies ready to save lives in battlefield situations.
Terumo BCT has entered into a business relationship with Kaneka to gain market authorization in the United States for the use of the Terumo BCT Spectra Optia Apheresis System with the Kaneka Liposorber LA-40S LDL Adsorption Column for cardiologists treating patients with high cholesterol.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 23, 2014 — Stentys presented final results from the APPOSITION IV study of its new self-apposing sirolimus-eluting stent (SES) during the Hotline session at the EuroPCR conference in Paris.
May 23, 2014 — The first drug-eluting balloon to go up for review by the U.S. Food and Drug Administration (FDA) will be discussed at the next Circulatory System Devices Panel of the Medical Devices Advisory Committee on June 12 in Germantown, Md.
May 23, 2014 — AliveCor Inc. announced studies presented at the Heart Rhythm Society's (HRS) 35th annual scientific sessions expand the growing body of clinical information evaluating the use of the AliveCor heart monitor.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

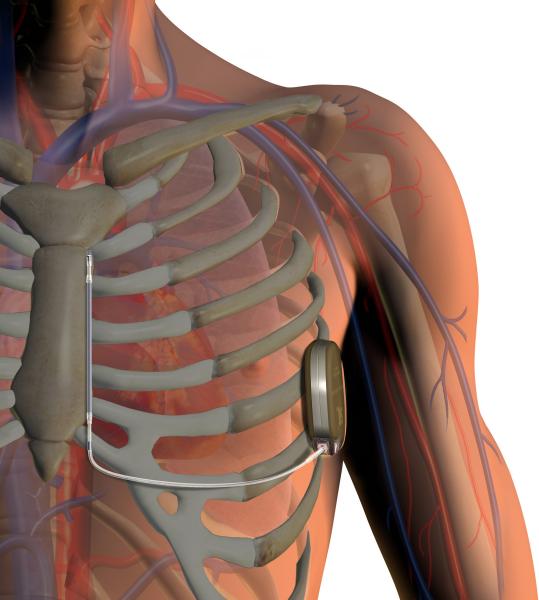
May 23, 2014 — Medtronic announced the results from the first prospective randomized clinical trial to show that Medtronic implantable cardioverter-defibrillators (ICDs) can safely extend detection times before triggering therapy in secondary prevention patients. The results of the PainFree SST sub-study were unveiled as a late-breaking presentation at the Heart Rhythm Society's (HRS) 35th annual scientific sessions.

Improved understanding of the molecular mechanism of atherogenesis will help identify disease-related pathways and biomarkers that may also help to predict the future risk in asymptomatic individuals. Novel technologies, such as high-throughput sequencing have enabled scientists to gain better insights into diseases and disease progression.
Extending the "Evolution of Visual Healthcare" to mobile devices, Agfa HealthCare launched its new Web-enabled mobile image management technology that brings the power of ICIS to iPhones, iPads, Web and Android mobile digital devices.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A study presented at Heart Rhythm 2014, the Heart Rhythm Society’s 35th Annual Scientific Sessions, reports significant gender and health insurance disparities in implantable cardioverter defibrillator (ICD) procedures.
Hospira Inc. issued a nationwide recall to the user level for one lot of Dobutamine Injection, USP, 250 mg, 20 mL, single-dose fliptop vial, (NDC 0409-2344-02), Lot 27-352-DK. (NDC and lot number can be found on the right-hand side of the primary label).
May 21, 2014 — Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) cleared the Somatom Force computed tomography (CT) system — the next generation in dual source CT.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
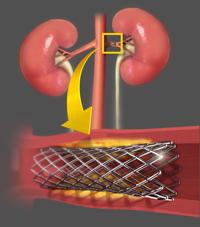
Patients with hypertension after renal artery stenting who do not respond to drug treatment may have another option.
May 21, 2014 — iRhythm Technologies Inc. announced that data from four studies presented at Heart Rhythm 2014, the Heart Rhythm Society's 35th Annual Scientific Sessions, support the use of the company's Zio Service to identify atrial fibrillation (AF) and other cardiac arrhythmias.
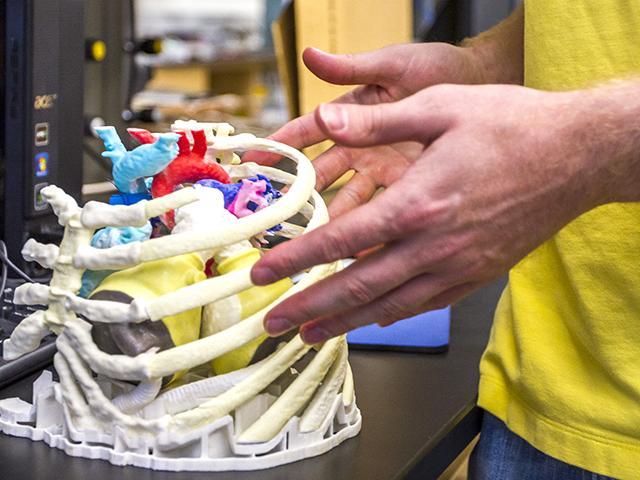
The FDA has recognized 3-D printing technology now exists to print medical devices and is gathering information regarding technical assessments that should be considered when it will inevitably begin reviewing these types of future FDA submissions.

 May 27, 2014
May 27, 2014