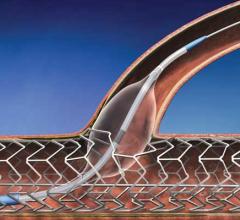
ClearFlow Inc. announced positive results from the Prevention of Retained Blood Outcomes Using Active Clearance Technology trial (PRO-ACT), a clinical study evaluating the use of PleuraFlow Active Clearance Technology System to prevent retained blood complications.
KLAS releases scores and analysis regarding solutions for electronic medical record (EMR) interoperability and health information exchanges (HIEs). This, to provide clarity into a market that is cluttered with competing claims.
Coquí RadioPharmaceuticals Corp. is working to become the first commercial producer of lifesaving medical isotopes in the United States. In 2012, Congress passed legislation making it a national priority to produce Molybdenum-99, an isotope necessary to detect a wide range of diseases, including cardiovascular disease and cancer.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Optimizing the treatment of coronary artery disease with a new foundation for future stent innovations, Medtronic Inc. announces CE mark and international launch of the Resolute Onyx Drug-Eluting Stent (DES). The first live patient implant of the Resolute Onyx DES occurred recently during the XII International Course of Endovascular and Myocardial Therapy in Madrid, Spain. The Resolute Onyx Drug-Eluting Stent is not approved in the United States.
St. Mary’s Health Care System installed two Vantage Titan 1.5T MR (magnetic resonance) systems from Toshiba America Medical Systems Inc. St. Mary’s utilizes the systems to enhance its cardiac imaging program and for advanced imaging studies that include myocardial perfusion and stress MR.
The actions — or inaction — of patients should be considered in programs designed to improve care and patient outcomes, according to a report released by the American College of Cardiology, American Heart Association, American Association of Cardiovascular and Pulmonary Rehabilitation, American Academy of Family Physicians and the American Nurses Association in collaboration with other professional organizations.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
LifeWatch AG is signing an agreement with Vital Connect Inc. to utilize their HealthPatch MD as a 1-lead ECG device in its cardiac monitoring business. LifeWatch and Vital Connect are working together to integrate the HealthPatch MD into LifeWatch's analytical mobile application and reporting systems with a view to bringing an integrated solution to market in the first half of 2015.
Catalist-listed QT Vascular Ltd. has acquired a novel technology platform called Java, and all associated intellectual property, which was developed independently in Israel.
Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
TIDI Products LLC has acquired CFI Medical Solutions (CFI) of Fenton, Mich., a diversified medical device manufacturer and engineering resource for hospitals, distributors and global original equipment manufacturers.

Viva Physicians, a not-for-profit organization in the field of vascular medicine and intervention, announce clinical trial results and other new happenings at Viva '14, hosted in Las Vegas, Nev.
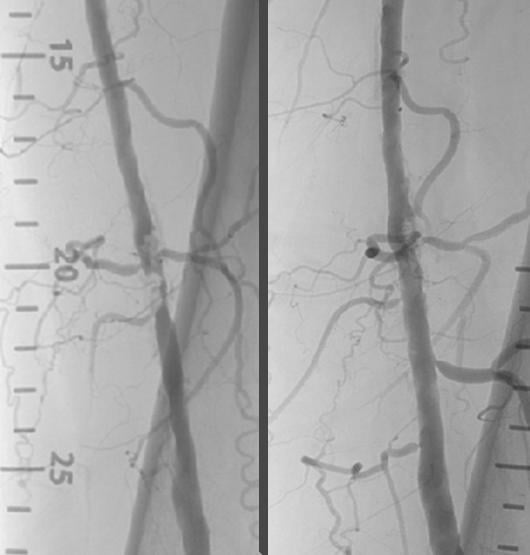
Shockwave Medical announced positive clinical results from Disrupt PAD, a single-arm multicenter study evaluating the safety and utility of Lithoplasty balloon catheters for the treatment of peripheral artery disease, at the Vascular Interventional Advances (VIVA) Annual Conference in Las Vegas, Nev.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Siemens announces the availability of its integrated mobile viewing application syngo.via WebViewer for all users of the newest syngo.via (VA30) image reading software. With the delivery of syngo.via VA30, users will automatically have the capability to easily and rapidly view images on mobile devices - within a hospital network or on the go.
Medic Vision Imaging Solutions Ltd. will showcase its SafeCT Enterprise solution at the upcoming Radiological Society of North America's (RSNA's) 100th Scientific Assembly and Annual Meeting, Nov. 30 - Dec. 5 in Chicago. SafeCT is a turnkey solution pioneered to revitalize existing computed tomography (CT) scanners with CT image enhancement for low-dose scans to enable low-dose imaging with high-quality image results for diagnosis. This provides hospitals and imaging centers with a cost-effective option in place of a costly scanner retrofit or replacement.
For the treatment of peripheral artery disease in leg arteries above the knee, the IN.PACT Admiral drug-coated balloon from Medtronic Inc. provided a consistently favorable treatment effect in patients with diabetes in a landmark study of the investigational medical device, which is under review for FDA approval.

 November 13, 2014
November 13, 2014