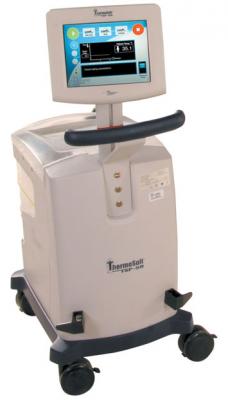
July 10, 2014 — Life Recovery Systems announced the U.S. Food and Drug Administration (FDA) has approved two investigational device exemptions (IDEs) to study the use of the ThermoSuit system in the treatment of heart attack and ischemic stroke. Patients in both trials will be cooled while under sedation, with the intent of reaching a state of therapeutic hypothermia in approximately 30 minutes or less.
"We are eager to see our rapid cooling treatment evaluated in victims of heart attack and stroke, as preliminary data predict that cooling will be most effective if it is provided close to the time of reperfusion," said Robert Schock, Ph.D., vice president of R&D for Life Recovery Systems.
The ThermoSuit system uses direct liquid contact with the patient to reduce core body temperature. A thin film of ice water is circulated over the patient's body until the temperature reaches a preset limit, at which point the water is automatically removed from the suit. The system is easily set up by nurses or technicians in minutes and cools to a therapeutic temperature of 33?C in about 30 minutes. The patient is then removed from the suit and remains cold for hours without the need for further maintenance, allowing unencumbered access for imaging or treatment in the cath lab.
For more information: www.life-recovery.com


 March 17, 2025
March 17, 2025