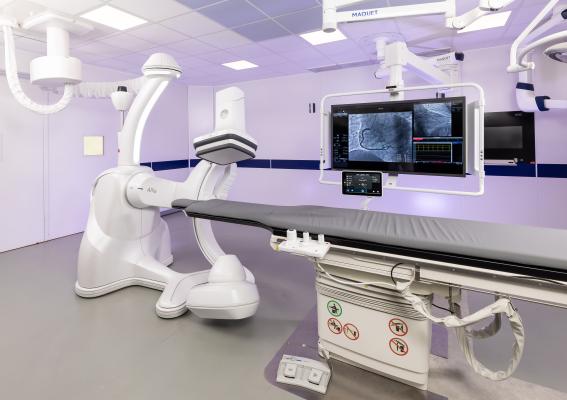
Building on the award-winning Allia platform for image guided therapies, GE HealthCare's new Allia IGS Pulse was designed to improve workflow for the diagnosis and treatment of cardiovascular diseases in interventional cardiology. (Photo: Business Wire)
October 16, 2023 — GE HealthCare (Nasdaq: GEHC) announced US FDA 510(k) clearance of Allia IGS Pulse - the latest addition to the company’s image guided system (IGS) offerings. Allia IGS Pulse features a new imaging chain engineered to provide exceptional imaging at the right dose for visible impact in complex cardiology interventions regardless of patient size.
In modern healthcare today, cardiovascular disease (CVD) continues to grow in prevalence, with CVDs being the leading cause of death globally.1 As clinicians work to treat CVDs, cardiology procedures continue to evolve as demand for minimally invasive surgery grows.2 GE HealthCare is committed to helping interventional cardiologists, vascular surgeons and other interventional specialists use image guidance technologies to their full potential by optimizing workflows and providing exceptional image quality to help clinicians provide quality care to their patients, while also enabling better clinical and operational outcomes.
According to a survey of interventionalists, nearly 50% of interventional procedures are performed at working positions where clinicians have poor access to user interface functions and display.3 Designed to address these challenges, the new Allia IGS Pulse provides a personalized workspace that meets the operator’s specific needs and preferences. As part of its new image chain, the system features the first monopolar x-ray tube used to capture images for interventional procedures. This new tube is powerful, yet quieter than normal conversation4 to optimize the operating environment during a procedure. The small footprint of the new tube also helps clinicians reach steep angulation for better understanding of coronary artery anatomy, even with the 30cm detector configurations. The latest version of MyIQ technology incorporated into the system allows clinicians to select their favorite image look from four different image styles with just one click for a tailored experience at no additional dose.5
In the treatment of complex cardiovascular diseases, image quality and optimized dose management regardless of patient size are also important to clinicians. With Allia IGS Pulse, interventionalists are able to get exceptional image quality for large and bariatric patients with a BMI of greater than 30. The system’s reduced pulse width and unmatched X-ray peak power helps to decrease motion blur for better visualization of moving elements such as vessels and devices. To relieve clinicians and technologists of the complex task of optimizing image quality and dose during procedures, Allia IGS Pulse takes AutoRight, the company’s intelligent image chain leveraging AI, a step further with AutoRight PLUS - the next generation of the automation platform. With AutoRight PLUS, the system now optimizes seven parameters in real time, including Focal Spot Shape. By removing the burden of manual adjustment during procedures, clinicians can stay focused on the procedure and treatment of their patients. To further optimize dose along the image chain, a unique suite of tools is also available to help support dose efficiency, dose reduction and dose awareness.
Since January 2023, Allia IGS Pulse has been in pilot operations at Clinique Pasteur - Toulouse in Toulouse, France.
“At Clinique Pasteur in Toulouse, we perform thousands of interventional cardiology procedures each year,” says Dr. Nicolas Dumonteil, Interventional Cardiologist, Clinique Pasteur, Toulouse.6 “As an operator of the Allia IGS Pulse system, I felt a significant and real improvement in the imaging quality, as well as a significant reduction of this noise in my daily procedures which gave me great confidence and comfort in the operating room. The system is also quite adaptable and versatile to all of my daily situations and procedures – from percutaneous coronary interventions (PCIs) to other complex and structural ones.”
“I saw a significant improvement in the image quality, especially with obese patients and complex angioplasties - where a good visibility of my guidewire, balloons and stents are particularly important,” says Dr Raphaël Philippart, Interventional Cardiologist, Clinique Pasteur, Toulouse.7
“Interventional cardiology procedures require exceptional image quality,” says Arnaud Marie – General Manager of Interventional for GE HealthCare. “I’m excited by the addition of Allia IGS Pulse to our interventional offerings because it addresses the very things clinicians continue to tell us present challenges in their day-to-day practice. By developing new features to further evolve our core platform, we’re helping to reduce complexity and improve the operating environment so that clinicians can have a personalized workspace that better enables them to keep their focus where it belongs – on their patients.”
For more information: www.gehealthcare.com
Find more TCT23 conference coverage here
References:
1 World Health Organization. Cardiovascular Diseases (CVDs). June 11, 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed February 2023.
2 Ali, Jason M, and Yasir Abu-Omar. “Minimally Invasive Cardiac surgery-a Fad or the Future?.” Journal of Thoracic Disease vol. 13,3 (2021): 1882-1885. doi:10.21037/jtd-2020-mics-12
3 47%, according to GE HealthCare sponsored blind survey conducted with 30 interventional cardiologists and interventional radiologists in the US and Europe.
4 System acoustic noise measured at 51.2dB(A) with 49 dB(A) background noise. Normal conversation is 60 dB (A).
5 Applicable only for cardiac protocols and not applicable with SV/SVV applications
6 Dr. Nicolas Dumonteil is a paid consultant for GE HealthCare and was compensated for participation in this testimonial. The statements by Dr. Nicolas Dumonteil described here are based on his own opinions and on results that were achieved in his unique setting. Since there is no “typical” hospital and many variables exist, i.e. hospital size, case mix, etc. there can be no guarantee that other customers will achieve the same results.
7 Dr. Raphaël Philippart is a paid consultant for GE HealthCare and was compensated for participation in this testimonial. The statements by Dr. Raphael Philippart described here are based on his own opinions and on results that were achieved in his unique setting. Since there is no “typical” hospital and many variables exist, i.e. hospital size, case mix, etc. there can be no guarantee that other customers will achieve the same results.


 October 24, 2025
October 24, 2025