July 10, 2014 — 480 Biomedical announced it has been awarded Phase II funding from the National Heart, Lung, and Blood Institute (NHLBI) to continue the development of a bioresorbable, self-expanding scaffold to treat pediatric pulmonary artery stenosis (PAS). The product aims to address the serious challenges in treating cardiovascular stenosis in children with growing blood vessels. The scaffold’s unique design has the strength of a balloon-expandable metal stent to keep vessels open for an extended period before safely resorbing. The scaffold can also be delivered through a small catheter, which is essential for treating a pediatric population. This latest $1 million round of funding will be used to further refine the design of the product and conduct preclinical testing.
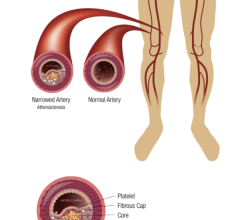
Metal stents, which are currently used in these procedures, are not well suited for pediatric patients since they become restrictive as the patient grows. Pediatric patients that are treated with metal stents often require re-intervention or surgical removal of the stent to address arterial obstruction and to reestablish blood flow.
“There has not been a significant advance in this area in decades,” said Robert J. Lederman, M.D., senior investigator and chief of cardiovascular intervention, division of intramural research, NHLBI. “480 Biomedical is the right team to develop a product that will help solve the critical challenge facing pediatric cardiologists in treating pulmonary artery stenosis. We are excited about the progress made to date and the overall important effort underway to improve care for the smallest, most vulnerable cardiology patients, who deserve our attention.”
480 Biomedical has developed a prototype of the pediatric scaffold that demonstrates acute strength similar to a metal stent, tissue absorption over the course of approximately one year, and minimally invasive delivery of the scaffold through a small flexible catheter. Under the provisions of the NHLBI contract, 480 Biomedical is responsible for the overall development of the pediatric scaffold and the NHLBI will undertake clinical study of the product.
“Tackling this complex problem successfully will have an important impact on the clinical approach to treating PAS, and potentially other pediatric cardiac and pulmonary conditions,” said Maria Palasis, Ph.D., executive vice president and chief technology officer, 480 Biomedical. “Our team is applying extensive knowledge and expertise from the development of our Stanza scaffold technology for peripheral vascular disease to transform care and improve outcomes for these young patients.”
The Stanza scaffold technology combines biocompatible materials with innovative engineering for optimal balance of radial force, flexibility and bioresorbability. This proprietary scaffold is also ideal for controlled delivery of drug for several months. It supports the lumen while healing occurs, resorbing in about a year. The strength of the scaffold, followed by its resorption, provides the benefits of scaffolding while avoiding the potential downsides of a permanent implant, including irritation, fracture and difficulty of retreatment.
For more information: www.480biomedical.com, www.nhlbi.nih.gov

 May 30, 2024
May 30, 2024