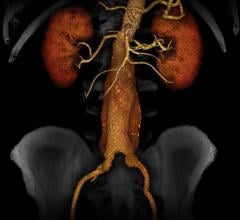
Fovia Medical, Inc. announced the introduction of an expanded high definition volume rendering software suite, featuring F.A.S.T. HDVR, at RSNA 2014.
Double Black Imaging released a new MRI safe display at RSNA 2014.
Tendyne Holdings Inc. announced that in November 2014, the Tendyne Transcatheter Mitral Valve Implantation system (TMVI) was successfully implanted in the first patient of the Tendyne Feasibility Study. This was the first patient enrolled in a three continent, multicenter trial, which aims to generate insight into the safety and performance of the Tendyne device in inoperable patients suffering from mitral regurgitation.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

For patients transcatheter aortic valve replacement (TAVR) patients whose anatomy prevents the use of femoral artery access, use of a transcaval access route (accessing the femoral vein and then tunneling into the aorta) may offer a new access route option.
The most accessed research studies in the Journal of the American College of Cardiology (JACC) in 2014 spanned many topics, including a blood test to rule out heart attack, an advisory about guidelines for preventing high blood pressure, cutting-edge research on a new class of cholesterol drugs and a study that found benefits in early surgery for mitral regurgitation.
Philips has signed an agreement with Image Stream Medical (ISM) that allows Philips to further expand its hybrid suite and interventional lab solutions with integrated video and live streaming capabilities. As part of the agreement, Philips has acquired a minority stake in ISM. Financial terms of the agreement were not disclosed.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Bayer unveiled version 2.5 of its Radimetrics Enterprise Platform at the 2014 Radiological Society of North America (RSNA) Scientific Assembly and Annual Meeting. The enhanced version has new features that can help radiologists and hospital administrators meet new 2015 U.S. Joint Commission Diagnostic Imaging Standards, providing information to help optimize radiation dose management and contrast dose analytics to advance image quality and patient care.
Vital Images Inc. received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Fenestrated Stent Planning workflow as part of its existing Endovascular Stent Planning (EVSP) application.
Ampronix reintroduced its Medvix line of near-patient surgical displays.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Ampronix announced the availability of its new LCD retrofit kit for the OEC 9800 mobile C-Arm.
Advanced Vascular Dynamics (AVD) announced U.S. Food and Drug Administration (FDA) clearance and CE mark for the RadAR vascular compression devices for post-procedure hemostasis of tibiopedal access sites, including the dorsalis pedis and posterior tibial arteries. The RadAR devices were previously cleared for use for post-catheterization radial artery hemostasis.
AliveCor Inc. announced the launch of its third-generation AliveCor Heart Monitor. Now users have the ability to identify and manage heart conditions with a U.S. Food and Drug Administration (FDA)-cleared electrocardiogram (ECG) monitor, anywhere and at anytime. The third-generation device is now 50-percent thinner and 40-percent lighter and was designed from first-hand customer feedback and in collaboration with global design firm IDEO.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

According to the Dell Global Technology Adoption Index, 96 percent of mid-size healthcare organizations surveyed are using or considering using cloud computing.
Veniti Inc. announced that it has enrolled the first United States patients in the Virtus trial of the Veniti VICI Venous Stent System.
With the introduction of the Aplio 300 and 500 Platinum Series from Toshiba America Medical Systems Inc., clinicians can now meet goals of the Centers for Medicare & Medicaid Services’ (CMS) Triple Aim.

 December 24, 2014
December 24, 2014