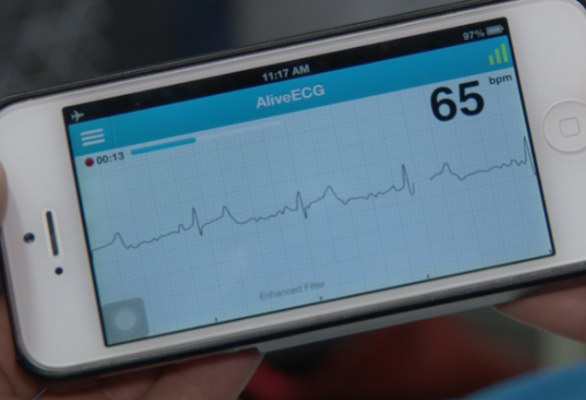
August 22, 2014 — AliveCor announced the U.S. Food and Drug Administration (FDA) has granted the company clearance for its algorithm to detect atrial fibrillation (AF), the most common form of cardiac arrhythmia. AliveCor’s free automated analysis process instantly detects if patients are experiencing AF through real-time electrocardiogram (ECG) recordings so physicians can intervene before potentially life-threatening conditions, like strokes, occur. Through AliveCor’s ECG analysis service, patients can confirm their results with a U.S. board-certified cardiologist or a personal physician. Now with the AliveCor Heart Monitor, free AliveECG app and AFib Detector, a serious medical condition can be detected entirely using a mobile device.
“The ability to automatically detect serious heart arrhythmia using mobile technology has the potential to save lives, reduce healthcare costs and allow patients and their caregivers to make informed decisions about cardiac care,” said Euan Thomson, president and CEO of AliveCor. “Having achieved clearance, we will work to incorporate the algorithm in our app and plan to make this available to customers during September.”
One in four adults over age 40 develop AF, making them five times more likely to have a stroke. AF can be hard to detect as symptoms, including heart palpitations, may be mild or non-existent. The AFib Detector represents a major step in the advancement of mobile health, allowing patients to instantly know if AF is present in their ECG.
“AliveCor is truly delivering on the promise of mobile health, placing medically relevant, intelligent tools in the hands of consumers worldwide,” said Trudie Lobban MBE, founder and CEO of AF Association & Arrhythmia Alliance. “We are excited about this milestone and look forward to using this technology to help those who suffer from arrhythmias identify when AF is present in an effective and efficient way so they can work with their doctors to manage their condition.”
The AliveCor Heart Monitor is intended to record, store and transfer ECG rhythms. It also displays ECG rhythms and detects the presence of atrial fibrillation when prescribed or used under the care of a physician. The AliveCor Heart Monitor is intended for use by healthcare professionals, patients with known or suspected heart conditions and health-conscious individuals. It is compatible with all iPhone models and most Android mobile devices. Users will continue to have the ability to access their data confidentially anytime, anywhere.
For more information: www.alivecor.com


 December 19, 2025
December 19, 2025 









