Catalist-listed QT Vascular Ltd. has acquired a novel technology platform called Java, and all associated intellectual property, which was developed independently in Israel.
Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for treating peripheral arterial disease (PAD) confirmed long-term patency for patients treated with Zilver PTX.
TIDI Products LLC has acquired CFI Medical Solutions (CFI) of Fenton, Mich., a diversified medical device manufacturer and engineering resource for hospitals, distributors and global original equipment manufacturers.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Viva Physicians, a not-for-profit organization in the field of vascular medicine and intervention, announce clinical trial results and other new happenings at Viva '14, hosted in Las Vegas, Nev.
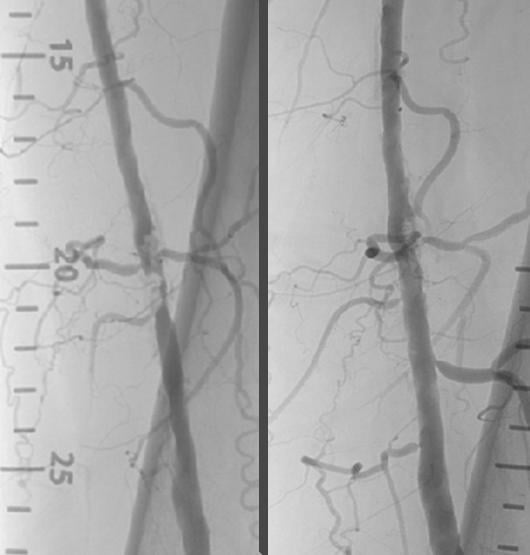
Shockwave Medical announced positive clinical results from Disrupt PAD, a single-arm multicenter study evaluating the safety and utility of Lithoplasty balloon catheters for the treatment of peripheral artery disease, at the Vascular Interventional Advances (VIVA) Annual Conference in Las Vegas, Nev.
Siemens announces the availability of its integrated mobile viewing application syngo.via WebViewer for all users of the newest syngo.via (VA30) image reading software. With the delivery of syngo.via VA30, users will automatically have the capability to easily and rapidly view images on mobile devices - within a hospital network or on the go.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medic Vision Imaging Solutions Ltd. will showcase its SafeCT Enterprise solution at the upcoming Radiological Society of North America's (RSNA's) 100th Scientific Assembly and Annual Meeting, Nov. 30 - Dec. 5 in Chicago. SafeCT is a turnkey solution pioneered to revitalize existing computed tomography (CT) scanners with CT image enhancement for low-dose scans to enable low-dose imaging with high-quality image results for diagnosis. This provides hospitals and imaging centers with a cost-effective option in place of a costly scanner retrofit or replacement.
For the treatment of peripheral artery disease in leg arteries above the knee, the IN.PACT Admiral drug-coated balloon from Medtronic Inc. provided a consistently favorable treatment effect in patients with diabetes in a landmark study of the investigational medical device, which is under review for FDA approval.
Merit Medical Systems Inc. settled a dispute regarding the distribution of the SecureLoc Safety Introducer Needle with Bard Access Systems. Under the terms of a settlement agreement, Bard Access Systems granted Merit a non-exclusive, worldwide license to manufacture and distribute the SecureLoc Safety Introducer Needle under its own trademark.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Lantheus Medical Imaging Inc. and Shine Medical Technologies Inc. announced that the companies have entered into a strategic agreement for the future supply of molybdenum-99 (Mo-99). The supply agreement marks Lantheus’ first with a prospective United States supplier of Mo-99.
Solid Water HE is the next generation of solid water phantoms by Gammex. It is designed for both therapy and imaging with improved uniformity and durability in mind.
Sorin Group, a global medical device company and a leader in the treatment of cardiovascular diseases, implanted the Solo Smart surgical replacement performed by David Heimansohn, M.D., at St. Vincent’s Heart Hospital in Indianapolis, Ind.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Backus Hospital, Hartford Hospital, The Hospital of Central Connecticut, MidState Medical Center, and Windham Hospital now gather and analyze dose data from all necessary modalities within their imaging departments to ensure an effective dose management program for their patients through the use of Sectra DoseTrack.
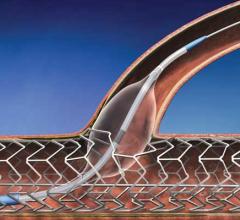
This multi-center pilot study focused on gathering data supporting the safety and performance of the Tack Endovascular System in 35 subjects with Critical Limb Ischemia (CLI) due to vascular disease below the knee.
Cardinal Health announced that its MynxGrip Vascular Closure Device recently received U.S. Food and Drug Administration (FDA) approval for use to close femoral veins. The MynxGrip device is now indicated for use to seal 5F, 6F and 7F femoral arterial and femoral venous access sites.

 November 12, 2014
November 12, 2014











