In July, The Heart Institute at Florida Medical Center became the first hospital in the state of Florida to offer the U.S. Food and Drug Administration (FDA)-cleared high sensitive Elecsys Troponin T Gen 5 blood test (Troponin T Gen 5 or TnT Gen 5) to aid in the diagnosis of heart attacks. The new nine-minute test offers faster answers clinicians can use when diagnosing heart attack.
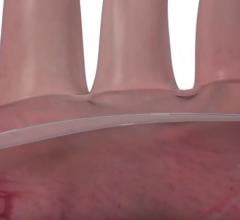
Protembis GmbH announced the first clinical applications of its ProtEmbo Cerebral Protection System to complement a transcatheter aortic valve replacement (TAVR) procedure. The ProtEmbo System is an intra-aortic filter device that deflects embolic material arising during TAVR away from the brain.
The U.S. Food and Drug Administration (FDA) has cleared TrueFusion, a new cardiovascular application from Siemens Healthineers that integrates ultrasound and angiography to guide cardiac teams when administering treatment for structural heart disease. Available on the new Release 5.0 of the Acuson SC2000 cardiovascular ultrasound system, TrueFusion is designed to maximize not only interventional cardiology procedures, but also routine diagnosis and follow-up of patients with structural heart disease.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 6, 2017 — Physicians identified a majority of patients with advanced heart failure as at high risk for ...
A new National Institutes of Health-funded hypertension trial will examine the possibility of using an emergency department setting to better identify people from central cities with limited access to other venues for medical care.
Minneapolis Heart Institute Foundation announced it has enrolled the first-in-the-world patient in a clinical study to evaluate a minimally invasive clip-based repair system made by Abbott for treating people with moderate or severe tricuspid regurgitation (TR). This study is the first application of this minimally invasive technology for use in the tricuspid heart valve, where currently there are no options for most patients. Paul Sorajja, M.D., performed this first-in-human procedure at Minneapolis Heart Institute at Abbott Northwestern Hospital.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Rambam Hospital in Haifa, Israel, recently became the first to use the CORolla device from Israeli start-up company CorAssist in a 72-year-old diastolic heart failure patient.
Abbott announced it has initiated a U.S. pivotal clinical study evaluating the safety and effectiveness of a modified version of its Amplatzer device designed to correct patent ductus arteriosus (PDA), a common congenital heart defect.
September 1, 2017 — Apixaban lowers the risk of stroke compared to warfarin in anticoagulation-naïve patients with ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
September 1, 2017 — Final results from the CASTLE-AF study show a 38 percent reduction in the composite of all-cause ...
September 1, 2017 — Biotronik recently announced data from the BIOFLOW-V randomized trial comparing Orsiro and Xience ...

September 1, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Cardiologists are constantly on the lookout for new methods to examine the heart using new imaging technology advances ...
Medtronic plc announced its intent to move forward with a new renal denervation pivotal trial following positive first results from a sham-controlled study in patients with high blood pressure. Investigators of the SPYRAL HTN-OFF MED Study found statistically significant and clinically important blood pressure reductions in the patients treated with renal denervation (RDN) across both office and ambulatory systolic and diastolic measurements. The data in the first 80 patients enrolled in the study at three months were presented in a late-breaking clinical trial session at the European Society of Cardiology (ESC) meeting, Aug. 26-30 in Barcelona, Spain and published simultaneously in The Lancet.
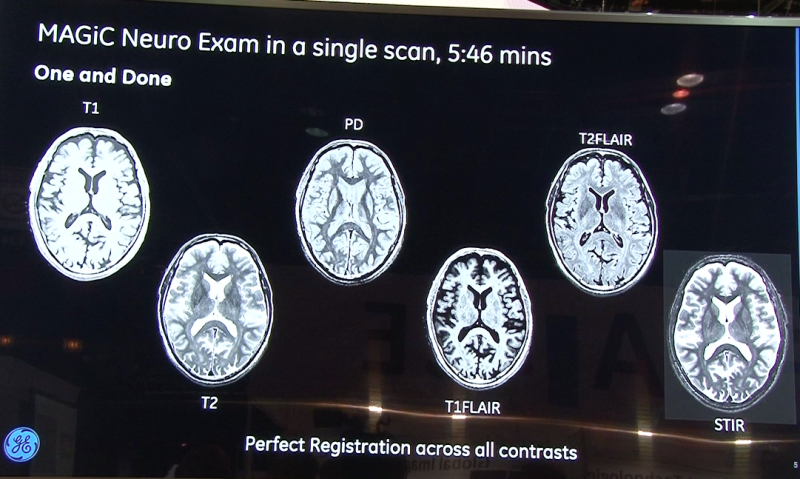
August 31, 2017 — The U.S. Food and Drug Administration (FDA) has granted market clearance for SyntheticMR’s SyMRI. The ...


 September 07, 2017
September 07, 2017