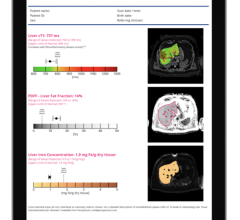
The MRI software enables several different contrasts to be obtained from one scan, shortening scan times.
August 31, 2017 — The U.S. Food and Drug Administration (FDA) has granted market clearance for SyntheticMR’s SyMRI. The magnetic resonance imaging (MRI) software delivers multiple, adjustable contrast images and quantitative data from a single five to six minute scan. The software reduces the need for several scans using different protocols, helping to reduce scan times and increases patient throughput.
The clearance means SyMRI IMAGE can be offered to new customer segments at hospitals and clinics on the U.S. market. The clearance is initially tied to MR scanners from GE Healthcare.
“The U.S. market alone is the largest MRI market globally. This FDA clearance is an important first step toward establishing SyMRI on this market. A natural next step is that we will now expand our marketing efforts in the U.S. We will also proceed with an application for regulatory FDA clearance for SyMRI Neuro,” said Stefan Tell, CEO SyntheticMR.
SyMRI also has European CE-marked clearance.
Read the related articles that include SyMRI, “Recent Advances in MRI Technology,” and “Software Advances in MRI Technology.”
For more information: www.syntheticmr.com


 January 21, 2026
January 21, 2026