September 7, 2017 — Protembis GmbH announced the first clinical applications of its ProtEmbo Cerebral Protection System to complement a transcatheter aortic valve replacement (TAVR) procedure. The ProtEmbo System is an intra-aortic filter device that deflects embolic material arising during TAVR away from the brain.
Darren Mylotte, M.D., and his team at Galway University Hospital, Ireland, performed the first-in-human procedure with the ProtEmbo System. He commented: “Over the next 10 years, TAVR procedures are expected to increase fourfold. With TAVR shifting to younger and lower-risk patients, cerebral protection becomes even more important. Clinical data from two recent studies of intermediate-risk patients undergoing TAVR suggest 30-day stroke risk as high as 5.5 percent. It is therefore critically important that we have a cerebral-focused protection device that will reduce the frequency of embolic events. The ProtEmbo System shows tremendous promise in reaching this clinical objective – first-in-human use of the device was safe and feasible.”
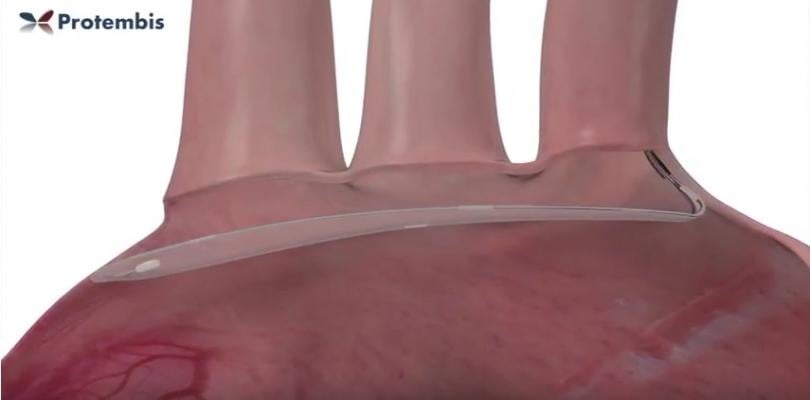
The objective of the current ongoing European trial is to demonstrate the safety and feasibility of the ProtEmbo System when used to provide embolic protection during TAVR. It was successfully deployed via the left radial artery across all three branches of the aortic arch for the duration of the TAVR procedures. The procedures were conducted under conscious sedation. On completion of the valve implant, the system was safely withdrawn without difficulty. None of the patients experienced a stroke event and there were no other device-related adverse events.
Features and benefits of the ProtEmbo include:
- Low-profile access via left radial artery (6Fr guiding sheath);
- Best access route: no interference with TAVR catheter or accessories;
- Simple, quick and reliable deployment;
- Complete coverage of all three aortic side branches;
- Deflection of microparticles, as tiny as 60 microns;
- Suitable for a wide variety of aortic arch anatomies; and
- Heparin coating for optimal biocompatibility.
Renu Virmani, M.D., president and medical director at CVPath Institute, Gaithersburg, Md., commented: “Our preclinical work with the ProtEmbo System prior to this clinical trial resulted in no safety or biocompatibility concerns. These initial findings from Europe now confirm our previous results. Despite the small pore size of the ProtEmbo System, there is no thrombus formation on the filter. This is promising because it may enable physicians to deflect even smaller particles away from the brain.”
Watch a video animation showing how the ProtEmbo system is deployed.
For more information: www.protembis.com


 November 14, 2025
November 14, 2025 









