At the 2018 European Society of Cardiology (ESC) Congress, cardiovascular technology startup Maisense debuted the Freescan telecardiology system that uses a portable device to measure blood pressure and record electrocardiogram (ECG) readings simultaneously.
This is a 360 degree photo inside a Detroit Fire Department ambulance equipped with a new Zoll X Series defibrillator ...
Philips announced that the European Society of Cardiology (ESC) has incorporated instantaneous wave-free ratio (iFR) into its updated revascularization guidelines. The new guidelines have provided the highest recommendation (class I A) for iFR alongside fractional flow reserve (FFR) for the objective assessment of the hemodynamic relevance of coronary lesions.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A newly developed rapid imaging protocol quickly and cheaply diagnosed heart ailments in patients in Peru, according to new research in Journal of the American Heart Association.
This is a view inside one of the 11 cath labs at Baylor, Scott, White Heart and Vascular Hospital in Dallas, Texas. This ...
This is a view from the control room for the dedicated cardiac 1.5T MRI system recently installed at Baylor, Scott ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Medtronic plc announced new findings from the CRYO4PERSISTENT AF clinical trial demonstrating improved quality of life, reduced symptoms from abnormal heart rhythms, and low incidence of reinterventions and repeat ablation procedures. The study evaluated patients with symptomatic persistent atrial fibrillation (AF) treated with the Medtronic Arctic Front Advance Cryoballoon, and the results were presented at the 2018 European Society of Cardiology (ESC) Congress, Aug. 25-28 in Munich.
A new study showed a change in the type of breathing tube paramedics use to resuscitate patients with sudden cardiac arrest can significantly improve the odds of survival and save thousands of lives. More than 90 percent of Americans who experience sudden cardiac arrest die before, or soon after, reaching a hospital.
iSchemaView announced that more than 575 stroke centers in 22 countries have selected the RAPID advanced imaging platform for cerebrovascular imaging, with 520 currently installed. RAPID technology assists physicians in the analysis of brain images using automated tools for CT ASPECTS, computed tomography (CT) angiography, CT perfusion, magnetic resonance (MR) diffusion and perfusion for more than 85,000 stroke cases per year. RAPID is also currently deployed in six multi-center clinical trials globally.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Blood pressure and cholesterol-lowering drugs continue to improve survival in patients with hypertension after more than a decade, according to late breaking results from the ASCOT Legacy study. The results were presented at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-29 in Munich, Germany, and published in The Lancet.
Acarix AB’s ultra-sensitive acoustic CADScor System for coronary artery disease risk assessment will be on display at the European Society of Cardiology (ESC) Congress 2018, Aug. 25-28 in Munich, Germany. The CADScor System has been validated clinically to rule out CAD with 96-97 percent negative predictive value. The device is gaining interest and increased use across Germany and Denmark, key Swedish hospitals are testing the system and expansion in other European countries is under way. At ESC 2018, both in the exhibition booth and at a special session Aug. 28, there will be opportunities to get first-hand information about the CADScor System and to meet experts with experience from using the system in clinical practice.
U.S. Expeditions and Explorations (USX) and Cardiac Insight Inc. announced the successful completion of an expedition on North America’s highest mountain, Denali, to gather data on sudden cardiac death (SCD) risk at high altitudes using the Cardea Solo device.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The National Institutes of Health announced in June it plans to end funding to the Moderate Alcohol and Cardiovascular Health (MACH) trial. The decision is based on concerns about the study design that cast doubt on its ultimate credibility. This includes whether the study would effectively address other significant consequences of moderate alcohol intake, such as cancer.
Cerner recently collaborated with Duke Clinical Research Institute (DCRI) to develop an atherosclerotic cardiovascular disease (ASCVD) Risk Calculator app. The app was designed as a tool to increase communication between the patient and their doctor about ways to live a healthier life and risk factors for heart disease and stroke.
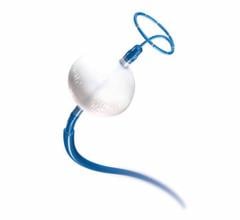
W. L. Gore & Associates Inc. (Gore) announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the Gore Molding & Occlusion Balloon. The compliant polyurethane balloon catheter is designed to assist in the expansion of self-expanding stent grafts or to temporarily occlude large-diameter vessels. The device also received approval from the Japanese Ministry of Health, Labour and Welfare, and receipt of CE Mark. It meets all endovascular aortic repair (EVAR) procedural requirements, according to Gore – a single balloon that replaces the need for multiple molding and occlusion balloons.


 August 30, 2018
August 30, 2018