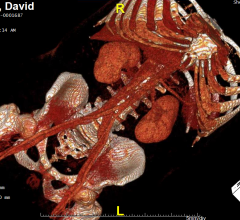
February 22, 2018 — AtriCure Inc. announced that it has launched the AtriClip FLEX•V Left Atrial Appendage (LAA) ...
Vascular closure device provider Cardiva Medical announced that the company has closed on $11 million in additional financing – bringing total equity and debt financing in the current round to $41 million. The additional financing exceeds previous commitments for this round and includes returning equity and debt investors – including PTV Healthcare Capital, Canepa Healthcare, and affiliates of Luther King Capital Management.
The U.S. Food and Drug Administration (FDA) issued the final rule on “Human Subject Protection; Acceptance of Data from Clinical Investigations for Medical Devices.” The rule updates the FDA’s standards for accepting clinical data from clinical investigations conducted both inside and outside the United States to help ensure the protection of human participants, and to help ensure the quality and integrity of data obtained from these clinical investigations.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Corindus Vascular Robotics Inc. announced today that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use of its CorPath GRX System in peripheral vascular interventions. The CorPath System is the first and only FDA-cleared medical device, according to the company, to bring robotic precision to both percutaneous coronary intervention (PCI) and peripheral vascular intervention (PVI) procedures.
Deep learning startup company Aidoc announced what it calls the world’s first and only comprehensive, full-body solution utilizing artificial intelligence (AI) to help analyze computed tomography (CT) scans, highlighting medical findings for radiologists. The workflow-integrated solution offers support for radiologists covering areas such as the head, c-spine, chest and abdomen.

One of the recent hot topics in interventional cardiology has been how operators can use new tools and techniques to ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 20, 2018 – Lexington Biosciences, Inc., a development-stage medical device company, announced the commencement ...
February 20, 2018 – LUMEDX Corporation, a top cardiovascular data intelligence company, will show off the latest in ...
A late-breaking analysis of the landmark COMPASS study was presented at the 2018 International Stroke Conference (ISC), showing that people with chronic coronary artery disease (CAD) and/or peripheral artery disease (PAD) taking Xarelto (rivaroxaban) had fewer ischemic strokes compared to those taking aspirin alone. This analysis, which specifically examined patients from COMPASS who experienced a stroke, also found high-risk patients taking Xarelto plus aspirin had the largest reductions in stroke.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 19, 2018 — The Detroit Medical Center’s (DMC) interventional cardiology team at Heart Hospital recently became ...
Claret Medical announced that since U.S. Food and Drug Administration (FDA) clearance in June 2017, its Sentinel Cerebral Protection System has been adopted by 50 U.S. centers as part of its controlled rollout. The company said that some institutions have adopted the device as an emerging standard of care in the U.S. to protect patients from the risk of stroke by capturing and removing debris associated with transcatheter aortic valve replacement (TAVR) before it travels to the brain. The novel system has been shown to significantly reduce the risk of stroke in the first three days after TAVR by more than 60 percent, according to Claret Medical.

For any cardiology department planning to upgrade its cardiovascular picture archiving and communication system (cardiac ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
February 16, 2018 — California’s Petaluma Health Center (PHC) was recently awarded a 2017 Healthcare Information and ...
The father of transradial artery access, Ferdinand Kiemeneij, M.D., Ph.D., interventional cardiologist, The Netherlands ...
DAIC Editor Dave Fornell previews the launch of augmented reality (AR) technology in the March/April 2018 issue of DAIC ...


 February 22, 2018
February 22, 2018