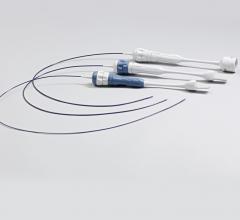
Ancora Heart Inc. announced positive clinical data from the company’s recently expanded U.S. early feasibility study evaluating the safety of the investigational AccuCinch Ventricular Repair System designed for the treatment of heart failure and functional mitral regurgitation (FMR). The data was presented at the 30th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, Sept. 21-25 in San Diego.
New data announced at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21-25 in San Diego, reinforce the safety, durability and consistency of the In.Pact Admiral drug-coated balloon (DCB) in real-world patients with peripheral arterial disease (PAD). Three-year real-world results from the full clinical cohort of the IN.PACT Global Study and one-year data from the Total IN.PACT pooled imaging and propensity analyses were presented at the TCT conference and the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual meeting, Sept. 22-26 in Lisbon, Portugal, respectively.
Corindus Vascular Robotics Inc. announced the first live transmission of a remote interventional procedure using the company’s CorPath platform was performed at the Transcatheter Cardiovascular Therapeutics (TCT) conference in San Diego on Sept. 22, 2018. The remote interventional procedure was broadcast live from Mayo Clinic to the TCT Main Arena. Utilizing CorPath GRX with developmental remote technology, combined with a telecommunications system to enable communication with the bedside staff, the operator at Mayo Clinic had the ability to remotely manipulate interventional devices within a porcine model's arteries while located in a different building.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
September 24, 2018 — Getinge is voluntarily initiating a worldwide recall involving a field correction of approximately ...
Osprey Medical announced a collaboration with GE Healthcare on Osprey’s Be Kind to Kidneys campaign. The campaign aims to increase awareness of strategies to help address acute kidney injury (AKI) following normal heart imaging procedures (angiograms) in patients with chronic kidney disease (CKD). As part of the campaign, Osprey Medical and GE Healthcare will sponsor a number of educational programs and seminars for healthcare professionals on how to address the risk of AKI.
At the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21-25 in San Diego, Siemens Healthineers will showcase new products that span the continuum of care in cardiology. Introducing solutions in both the laboratory and imaging spaces, Siemens Healthineers will debut a new ultrasound real-time intra-cardiac echo (ICE) catheter and High-Sensitivity Troponin I assays. The company is also a sponsor of the Interventional Imaging and Endovascular Pavilions at TCT, supplying hands-on workshops and simulations for continuing education.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

October 4, 2018 – The Cardiovascular Research Foundation (CRF) had 15 late-breaking trials and 12 late-breaking clinical ...
At the Transcatheter Cardiovascular Therapeutics (TCT) annual meeting, Sept. 21–25 in San Diego, Philips is showcasing its latest solutions in interventional cardiology. These solutions combine imaging systems, planning and navigation software, and specialized diagnostic and therapeutic devices, helping clinicians conduct procedures that are fast, efficient and personalized.
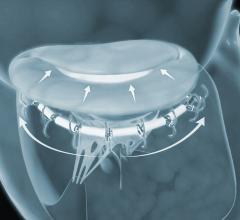
Cardiac Dimensions announced the company has randomized its first patient in the CARILLON Pivotal Trial.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
September 19, 2018 — Abiomed Inc. announces its initiatives at the 30th Transcatheter Cardiovascular Therapeutics (TCT) ...
September 19, 2018 — DiA Imaging Analysis Ltd. (DiA), a provider of artificial intelligence (AI)-powered ultrasound ...
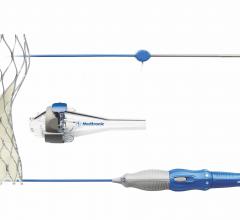
The U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) to initiate a new single-arm study of Medtronic’s CoreValve Evolut transcatheter aortic valve replacement (TAVR) system in patients with bicuspid aortic valves. All patients in the study will be at low risk of surgical mortality. Medtronic separately received FDA approval for revised commercial labeling for the CoreValve Evolut TAVR system that removed a precaution for the treatment of bicuspid severe aortic stenosis patients deemed at intermediate or greater risk for surgical aortic valve replacement.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
William O’Neill, M.D., outlines his recent clinical publication of AMICS patients from the Impella Quality (IQ) database ...
Raymond Kwong, M.D., MPH, from the Harvard Medical School recently presented his findings on a study of how single-center stress cardiovascular magnetic resonance (CMR) research studies have shown excellent values in management of patients with suspected ischemia. Kwong, who is also a member of the Society for Cardiovascular Magnetic Resonance (SCMR) presented his findings at the 2018 European Society of Cardiology (ESC) Congress, Aug. 25-29 in Munich, Germany.
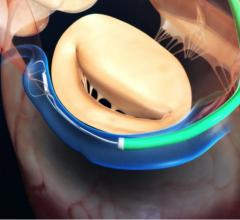
September 17, 2018 — 4C Medical Technologies Inc. announced that its AltaValve transcatheter mitral valve replacement ...

 September 24, 2018
September 24, 2018