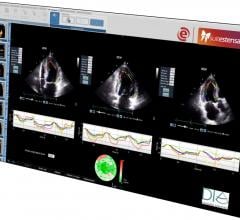
DiA Imaging Analysis has partnered with the Italian healthcare IT company Ebit (Esaote Group), to offer DiA’s LVivo Cardiac Toolbox as an integrated part of Ebit's Suitestensa CVIS (cardiovascular information system). The LVivo Cardiac Toolbox is designed to analyze cardiac ultrasound images based on more objective and reproducible information, as opposed to manual measurement or visual analysis methods that are currently being used.
HonorHealth Research Institute announced the first patients have been enrolled in the SynIVUS-DAPT Study. The clinical study is designed to test outcomes of decreasing the amount of time patients with a high risk of bleeding take antiplatelet medications after receiving a drug-eluting coronary stent.
CardioFocus Inc. announced the European CE Mark approval of the HeartLight X3 Endoscopic Ablation System.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Over the last 40 years, despite multiple advancements in percutaneous coronary interventions, calcified lesions remain a ...
For the first time in the United States, doctors with the American Heart Association (AHA) have outlined best practices for cardiologists to evaluate and manage patients who have heart attacks with no significant signs of coronary artery disease — a condition known as myocardial infarction with non-obstructive coronary arteries (MINOCA). The new document, published in the March 27 issue of Circulation,1 aims to help physicians better recognize patients with this condition, to avoid common misdiagnoses and streamline care. It is especially important for women, who represent a disproportionate number of MINOCA cases.

As we strive to process today’s successive news cycles involving negative reports about immigration, it is easy for many ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 15, 2019 – Intact Vascular Inc. received U.S. Food and Drug Administration (FDA) market clearance for the Tack ...
April 15, 2019 — Biotronik began its U.S. commercial launch of the PK Papyrus covered coronary stent system for use in ...
Despite national guidelines indicating statins can lower risk of heart attack and stroke, many patients who could benefit do not take them. More than half of eligible patients say they were never offered the cholesterol-lowering drugs; the experience of side effects or fear of side effects were reasons for stopping or refusing statins, according to new research in Journal of the American Heart Association.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
This week, cardiologists learned for the first time they have been examining black holes for decades and did not know it ...
Smart speakers customarily used in your living room can act as an aid to physicians in hospital operating rooms, according to new research presented at the Society of Interventional Radiology’s (SIR) 2019 Annual Scientific Meeting, March 23-28 in Austin, Texas. Smart speakers, such as the Amazon Echo and Google Home, offer a conversational voice interface that allows interventional radiology (IR) physicians to ask questions and retrieve information needed for their patient treatments without breaking sterile scrub.
According to a new research study, straight tip guidewires, with a current share of more than a third of the total market value, will retain the dominant position in neurovascular guidewires market through 2028. The report from Future Market Insights notes that aneurysms and intra-and extra-cranial angioplasty reflect highest applicability of neurovascular guidewires.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A program designed to help heart attack patients with the transition from hospital to outpatient care can reduce readmissions and deaths and increase the number of patients keeping follow-up appointments, a new study suggests. Findings from the Sanger Heart and Vascular Heart Care Navigation Team study were presented at the American College of Cardiology’s Cardiovascular Summit, Feb. 14-16 in Orlando, Fla. The conference brings together top experts to discuss and review innovative, relevant cardiovascular management and leadership strategies.
DiA Imaging Analysis announced the launch of LVivo SAX, a cardiac analysis tool that helps clinicians quickly and accurately interpret ultrasound images and assess heart functionality among patients suspected of suffering from acute coronary syndrome (ACS).
Rolling Stones frontman Mick Jagger is reportedly recovering after undergoing a transcatheter aortic valve valve replacement (TAVR) procedure at a New York hospital.

 April 16, 2019
April 16, 2019
















