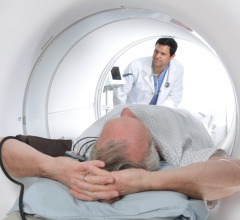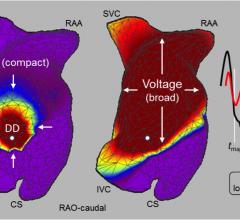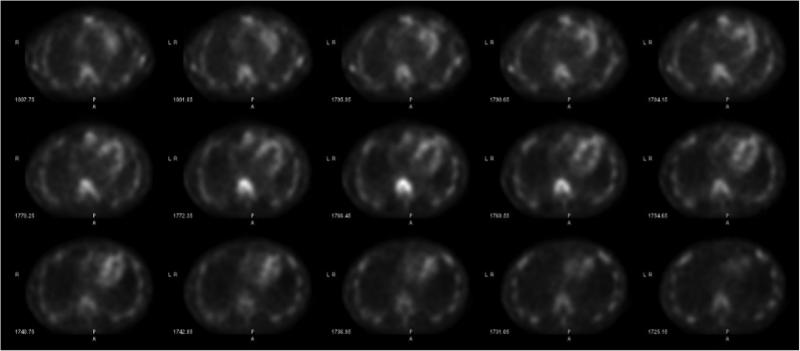
Cardiac amyloidosis is a highly morbid and underdiagnosed infiltrative cardiomyopathy that is characterized by the ...
Medical device startup company Filterlex Medical recently completed a series A financing round, raising a total of $3 million for its Captis embolic protection device for reducing the risk of stroke and other complications during catheter-based structural heart procedures. Captis won Best Innovation Award at the EuroPCR 2019 innovation competition in Paris and was awarded a grant of $200,000 by the Jon DeHaan foundation.
A new method of evaluating irregular heartbeats outperformed the approach that’s currently used widely in stroke units to detect instances of atrial fibrillation (Afib).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Ron Blankstein, M.D., director of cardiac computed tomography, Brigham and Women's Hospital, and associate professor of ...
Quynh Truong, M.D., MPH, associate professor of radiology and medicine at Weill Cornell and director of cardiac CT ...
Low doses of radiation equivalent to three computed tomography (CT) scans, which are considered safe, give cancer-capable cells a competitive advantage over normal cells in healthy tissue, scientists have discovered. Researchers at the Wellcome Sanger Institute and the University of Cambridge studied the effects of low doses of radiation in the esophagus of mice.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
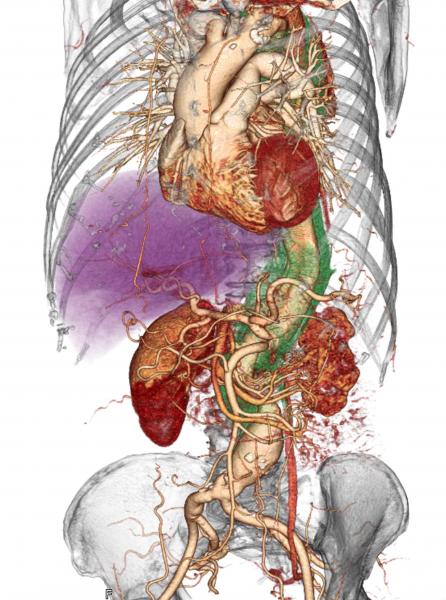
When a patient comes to the hospital with acute type A aortic dissection (ATAAD), a tearing of the inner lining of the aorta, surgeons typically perform an immediate open repair to prevent death from aortic rupture.
On June 30, 2019, the Centers for Medicare & Medicaid Services (CMS) announced the Johns Hopkins University School of Medicine has been designated a qualified provider-led entity (qPLE). This allows Johns Hopkins to develop criteria that meet the requirements of the federal Protecting Access to Medicare Act (PAMA) of 2014 when ordering diagnostic imaging tests such as computed tomography (CT) scans, magnetic resonance imaging (MRI) scans and nuclear imaging in the emergency department and ambulatory settings.
A new analysis published by The Sage Group LLC concludes that the all-cause cost of critical limb ischemia (CLI) exceeds $200 billion in the United States.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) announced the 2020 Experiential Learning Program site visit proposal solicitation period. The 2020 Fall ELP submission period is open from Monday July 8, 2019 – Thursday, August 8, 2019, at 12 p.m. EST. The ELP is intended to provide a formal training mechanism for regulatory review staff to visit research, clinical, manufacturing and healthcare facilities to observe how medical devices are designed, developed and utilized.
Abbott has received U.S. Food and Drug Administration (FDA) approval for the most advanced MitraClip heart valve repair device to treat mitral regurgitation. The latest approval for the fourth-generation MitraClip device, MitraClip G4, puts new enhancements into the hands of physicians across the U.S. by delivering an expanded range of clip sizes, an alternative leaflet grasping feature and facilitation of procedure assessment in real time to offer doctors further options when treating mitral valve disease.
Elevated blood pressure and cholesterol levels in young adulthood may lead to an increased risk of heart disease later in life, regardless of later-in-life exposure to these risk factors, according to research published in the Journal of the American College of Cardiology.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
NorthStar Medical Radioisotopes LLC announced completion of construction on its 20,000-square-foot molybdenum-99 (Mo-99) processing facility in Beloit, Wis., with equipment installation currently underway. Establishing this processing facility is part of NorthStar’s staged development and dual processing pathway approach to expanding current capacity and efficiencies in Mo-99 production.
Acutus Medical announced the publication of the UNCOVER AF study in Circulation: Arrhythmia and Electrophysiology. The study demonstrated 73 percent single-procedure freedom from atrial fibrillation (AF) at 12 months with the use of Acutus' AcQMap advanced cardiac imaging and mapping system. Acutus develops electrophysiology (EP) technology solutions built into an open platform EP suite of products that enable personalized and adaptive approaches to therapy.
Edwards LifeSciences is recalling the IntraClude Intra-Aortic Occlusion Device due to a risk of balloon rupture during use, which may add time to the procedure and compromises the safety of the patient.

 July 22, 2019
July 22, 2019