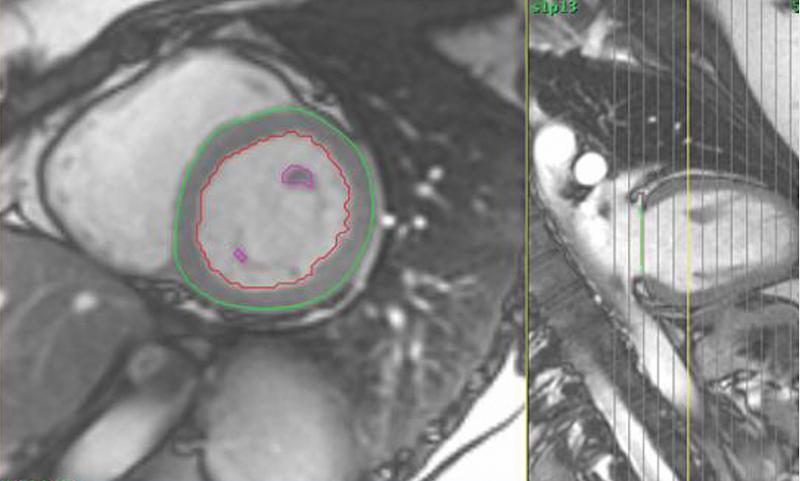
October 29, 2020 — Contrast agents used to improve views of the heart on magnetic resonance imaging (MRI) carry a very ...
Oct. 29, 2020 — Boston Scientific initiated the CHAMPION-AF clinical trial to evaluate the safety and efficacy of the ...
October 28, 2020 — Northwestern Memorial Hospital is the first hospital in the United States to purchase Caption Health ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The September-October 2020 digital edition of Diagnostic and Interventional Cardiology (DAIC) magazine include links to ...
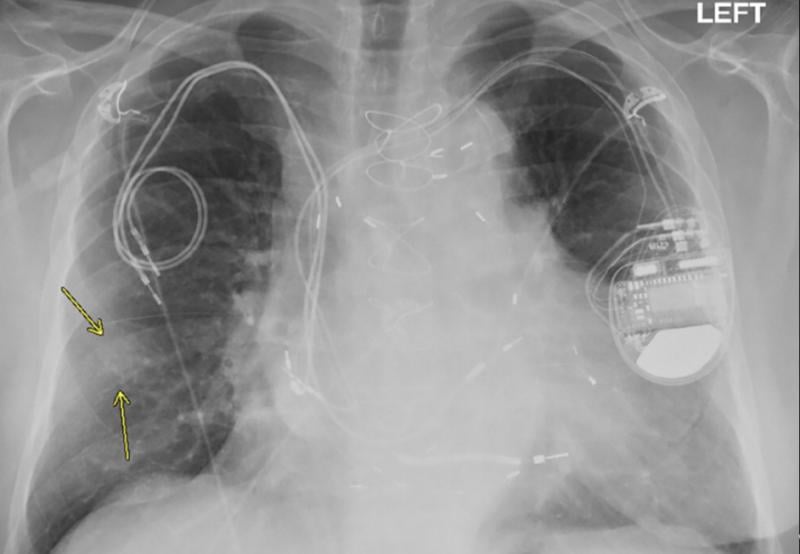
October 27, 2020 – Magnetic resonance imaging (MRI) examinations can be safely performed in patients with non-MR ...

October 26, 2020 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed for an all-in-one ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 26, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic Abre venous self-expanding ...
October 23, 2020 — Positive results were reported on the first 230 patients enrolled in its FLASH study, a real world ...
October 23, 2020 – Results from the randomized controlled TARGET FFR trial show that while a physiology-guided ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
October 22, 2020 – In the FORECAST randomized clinical trial, the use of fractional flow reserve (FFR) derived from ...

October 17, 2020 – In a surprise to many, a randomized clinical trial found that drug-eluting stents (DES) with durable ...
October 22, 2020 – For patients undergoing percutaneous coronary intervention (PCI), treatment with the nanotechnology ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Chuck Simonton, M.D., chief medical officer at Abiomed, discusses some of the new technologies and clinical trials the ...
Chuck Simonton, M.D., chief medical officer at Abiomed, explains when advanced hemodynamic support is required in COVID ...
Dean Kereiakes, M.D., medical director, The Christ Hospital Heart and Vascular Center, medical director, The Christ ...

 October 29, 2020
October 29, 2020














