December 21, 2020 – A proposed ASTM International standard will answer the need for a standardized test method that ...
December 21, 2020 — U.S. Food and Drug Administration (FDA) approved updated labeling December 17 for Abbott's HeartMate ...
December 16, 2020 — AI Medic Inc. announced that it has obtained official product certification from the NIDS (National ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
December 16, 2020 — Efemoral Medical announced the first-in-human (FIH) use of the its Efemoral bioresorbable vascular ...
December 16, 2020 — Corindus, A Siemens Healthineers company and a leading developer of vascular robotics, began its ...
December 16, 2020 - Intelerad Medical Systems, a provider of enterprise imaging workflow solutions, has acquired ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
This is an example of the Medis Medical Imaging Quantitative Flow Ratio (QFR) system that offers a fractional flow ...
This is an example of the Siemens Corindus CorPath Cath lab robotic system being used for a percutaneous coronary ...
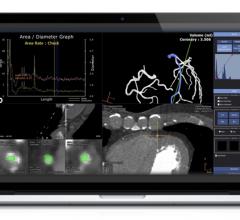
December 16, 2020 – Acist Medical Systems Inc. announced a formal distribution partnership with Medis Medical Imaging to ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...
To improve efficiencies in your cardiology department, you need technology to streamline workflows, reduce redundant ...
December 14, 2020 - Foldax Inc. today announced that the U.S. Food and Drug Administration (FDA) has granted an ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

December 14, 2020 — The U.S. Food and Drug Administration (FDA) issued the first emergency use authorization (EUA) for ...
December 9, 2020 — SoniVie, an Israeli company developing the Therapeutic Intra-Vascular Ultrasound (TIVUS) System to ...
Todd Hurst, M.D., a cardiologist at Banner University Medicine Heart Institute, and an associate professor at the ...


 December 21, 2020
December 21, 2020