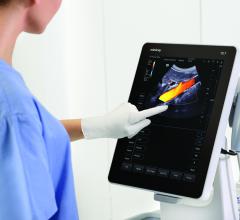
Konica Minolta Medical Imaging announced new Blue Moon Lifecycle Solutions designed to help customers minimize downtime, maximize productivity, and eliminate risk for the lifetime of their handheld Sonimage P3 and portable Sonimage HS1 ultrasound systems. The company will introduce the new Blue Moon Lifecycle Solutions at the Association for Medical Imaging Management (AHRA) annual meeting, July 20-22 in Las Vegas.
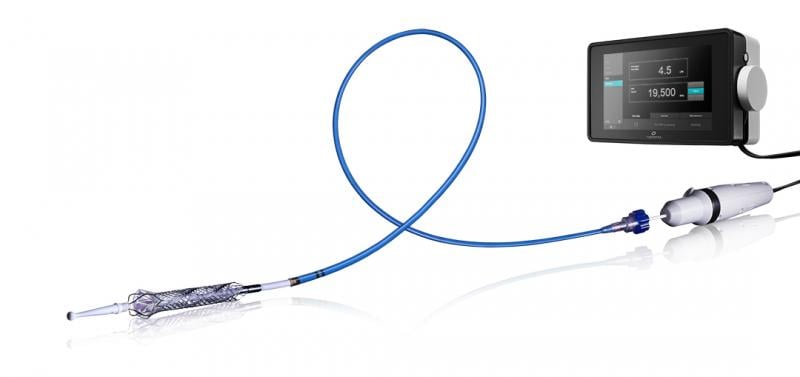
Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval, permitting sale in the European Union and other international countries. Approval was based on data from the first 30 patients enrolled in the HeartMate PHP SHIELD I CE Mark Trial examining use of PHP to support patients undergoing a high-risk percutaneous coronary intervention (PCI) procedure. Data from all 50 patients enrolled in this study will be presented later in 2015.
Biotronik announced the completion of the BIOVALVE first-in-human trial for its new transcatheter aortic valve. Study doctors successfully implanted the device in patients suffering from severe symptomatic aortic stenosis. The study, which established the transcatheter aortic valve implantation (TAVI) device's early safety at 30 days, was conducted at the University Heart Center Hamburg-Eppendorf (UKE), Germany.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Mindray announced the release of its TE7 Touch Enabled Ultrasound System, which supports rapid and confident evaluation in multiple point-of-care (POC) settings. The intuitive tablet-like operation, high image quality with one-touch image optimization, and exam presets improve both diagnostic confidence and efficiency.
On July 8, the Centers for Medicare and Medicaid Services (CMS) released the first proposed update to the physician payment schedule since the repeal of the Sustainable Growth Rate in April. The proposal includes a number of provisions focused on person-centered care, and continues the Obama administration’s commitment to transform the Medicare program to a system based on quality and healthy outcomes.
Rivanna Medical announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market Accuro, a handheld and untethered smart phone-sized ultrasound device. Accuro is designed to guide spinal anesthesia with automated 3-D navigation technology in addition to ultrasound imaging of abdominal, musculoskeletal, cardiac and peripheral vascular anatomies.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
PHS Technologies Group LLC, a division of PACSHealth LLC, announced that it will integrate VirtualDose CT (computed tomography) software from Virtual Phantoms Inc. into their DoseMonitor product.

Hospitals performing carotid artery stenting vary considerably in rates of in-hospital stroke or death, according to a study published in JACC: Cardiovascular Interventions. Those rates can range from 0 to 18 percent overall, and from 1.2 to 4.7 percent when accounting for variation in health of patients at admission.

Edwards Lifesciences Corp. announced that it has agreed to acquire CardiAQ Valve Technologies Inc., a privately held company and developer of a transcatheter mitral valve replacement (TMVR) system.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

The U.S. Food and Drug Administration (FDA) is alerting healthcare providers and patients of reports of patient deaths and other serious adverse events associated with the use of the Lariat Suture Delivery Device used for minimally invasive surgical closure of the left atrial appendage (LAA)

The U.S. House of Representatives July 10 passed its version of the 21st Century Cures Act (H.R. 6), designed to improve the U.S. healthcare innovation infrastructure. It was approved in a bipartisan vote of 344-77. The bill calls for providing resources to researchers working on next-generation medical devices and therapies. The legislation is aimed at addressing concerns that U.S. healthcare innovation is lagging behind the rest of the world due to large amounts of time-consuming and expensive regulatory oversight requirements that some feel are stifling innovation and the ability of startup companies to bring new products to market.
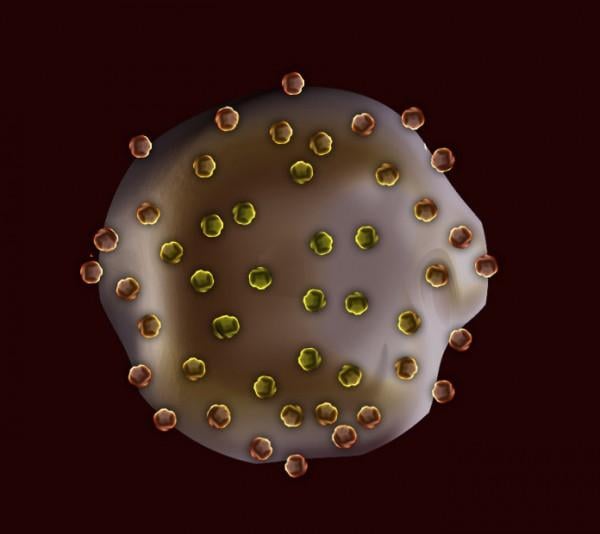
Researchers have created tiny gel particles that can perform the same essential functions as platelets. The particles could one day be used to control excessive bleeding following traumatic injury, or in individuals with impaired clotting due to an inherited condition or as a result of certain medications or chemotherapy.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

Wearable sensors are expected to play a big role in healthcare in the coming years, according to Verizon, as data from these devices are integrated into patient electronic medical records. They offer a big-picture view of a patient's health, beyond a yearly checkup. These devices are also expected to play a major role as healthcare organizations look for new ways to engage patients electronically to meet new federal health IT requirements.
Opsens Inc. announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the OptoWire and OptoMonitor, its products developed to measure fractional flow reserve (FFR). This measure is used to optimize the diagnostic and guide the treatment of patients with coronary heart disease.
The Centers for Medicare & Medicaid Services (CMS) and the American Medical Association (AMA) announced efforts to help physicians prepare for the nationwide switch from ICD-9 to ICD-10 ahead of the October 1 deadline. In response to requests from the provider community, CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set for medical diagnoses and inpatient hospital procedures.

 July 15, 2015
July 15, 2015