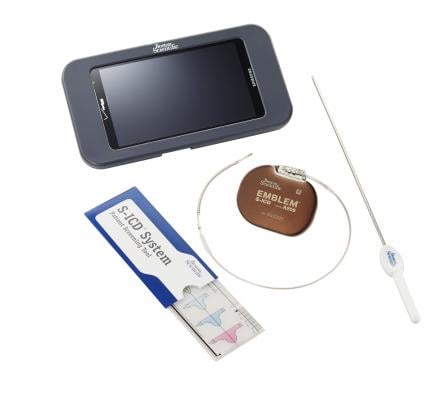
March 19, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) and CE Mark approval of the Emblem Subcutaneous Implantable Defibrillator (S-ICD) system. The Emblem S-ICD system is a treatment option that provides protection for patients at risk of sudden cardiac arrest (SCA), yet leaves the heart and vasculature untouched, minimizing the risk of complications associated with conventional transvenous implantable cardioverter-defibrillators (TV-ICDs). A controlled and limited market release has begun in a small number of European centers, with a broad European launch scheduled for May 2015 and subsequent U.S. launch planned for the third quarter of 2015.
Unlike traditional ICDs that require placement of at least one lead in or on the heart, the S-ICD System is implanted just under the skin and provides the patient the same protection from cardiac arrest without invading the heart and blood vessels. Leads in the heart may be associated with infrequent but serious complications — including lead displacement, fracture and systemic blood infections or the need for lead extraction — which may lead to hospital readmission, increased mortality and associated costs.
The new-generation Emblem is 19 percent thinner and is projected to last 40 percent longer than the previous S-ICD System. These improvements will further improve patient comfort and cosmetic outcomes while reducing the number of times the device will require replacement. The system is also enabled for remote patient management through the Latitude NXT Patient Management System for increased patient convenience.
For more information: www.sicdsystem.com


 January 13, 2026
January 13, 2026 









