Boston Scientific Corp. announced the acquisition of Securus Medical Group Inc., a privately-held company that has developed a thermal monitoring system for the continuous measurement of esophageal temperature. Boston Scientific has been an investor in Securus since 2016, and the transaction price for the remaining stake not already owned consists of $40 million in cash up-front, as well as up to $10 million in contingent payments based on regulatory achievements and commercial milestones.
April 4, 2018 — The U.S. Food and Drug Administration (FDA) has cleared the Somatom Edge Plus computed tomography (CT) ...
April 3, 2018 — Following the unprecedented Gore REDUCE Clinical Study conclusion that closure of patent foramen ovale ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
GE Healthcare announced the sale of its Value-Based Care Division — including its software assets for Enterprise Financial Management, Ambulatory Care Management and Workforce Management — to private equity investment firm Veritas Capital. The sale, expected to be completed in third quarter 2018, was valued at $1.05 billion in cash.

Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...
Abiomed Inc. announced that it received U.S. Food and Drug Administration (FDA) Pre-Market Approval (PMA) for its Impella CP heart pump with SmartAssist, utilizing an optical sensor. The company said these advances in technology and software are designed to improve productivity, ease of use and patient management to ensure optimal patient care.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Revised guidelines for the nonsurgical, image-guided interventional treatment of acute ischemic stroke will ensure patients receive the right treatment at the right time. The updated guidelines, endorsed by the Society of Interventional Radiology (SIR) and 11 other medical societies, were published in the April edition of SIR’s peer-reviewed journal, the Journal of Vascular and Interventional Radiology (JVIR).
Philips in partnership with Innovative Imaging Technologies (IIT) announced an industry-first integrated tele-ultrasound solution on Philips’ Lumify portable ultrasound system powered by IIT’s Reacts collaborative platform. This innovation connects clinicians around the globe in real time by turning a compatible smart device into an integrated tele-ultrasound solution, combining two-way audio-visual calls with live ultrasound streaming.
March 30, 2018 — The Marcus Foundation has donated $15 million to establish the Marcus Stroke Network, a coordinated and ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
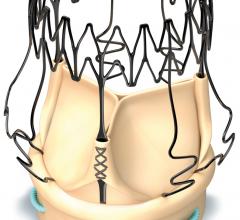
LivaNova PLC announced the first patient enrollment in the Perceval Valve Clinical study for Chinese Registration (“PERFECT”) Trial. The study is a pre-market, prospective, single-arm trial. The PERFECT trial is being conducted to demonstrate the safety and effectiveness of the Perceval sutureless aortic heart valve when used to replace a diseased native or malfunctioning prosthetic aortic valve in the indicated Chinese population for tissue heart valve replacement.
March 30, 2018 — Bariatric surgery is predicted to cut in half the risk of premature heart disease and stroke in teens ...

Magnetic resonance imaging (MRI) for cardiac assessment provides a radiation-free alternative to other commonly used ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
March 29, 2018 — The Aquilion One/Genesis Edition computed tomography (CT) system from Canon Medical Systems gives ...
Abbott announced the company has initiated the landmark GUIDE-HF clinical trial using the CardioMEMS HF System. The GUIDE-HF trial will study whether the CardioMEMS device can improve survival and quality of life for people living with New York Heart Association (NYHA) Class II - IV heart failure.
Population health enablement company Higi announced their commitment to implement the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure guidelines across the 11,000 U.S. Food and Drug Administration (FDA)-cleared Higi stations nationwide. Higi said it will use its stations at retail stores and community locations to engage consumers in their health, helping them understand their biometric measurements and connecting them with the appropriate care for earlier intervention and better management of hypertension treatment plans.


 April 04, 2018
April 04, 2018