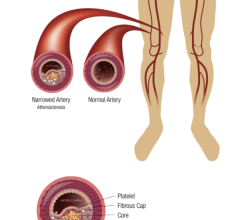
July 12, 2013 — Micell Technologies Inc. received CE mark approval for its MiStent sirolimus-eluting absorbable polymer coronary stent system (MiStent SES). The company is introducing a thin-strut stent that features elimination of the coating from the stent in 45-60 days and the complete absorption of the polymer coating within 90 days. The MiStent SES is unique in providing local drug delivery both during and after the period of polymer absorption, thereby eliminating long-term polymer exposure, a potential cause of delayed healing and late adverse events.
Arthur J. Benvenuto, CEO of Micell, said, "The MiStent SES brings a new paradigm of safety without compromise to efficacy or deliverability. With polymer absorption faster than any other DES [drug-eluting stent] currently available, we believe the MiStent SES provides a long-term safety profile of a highly deliverable bare metal stent."
The MiStent SES approval is supported by in-depth clinical analysis from the DESSOLVE I and DESSOLVE II clinical trials. The DESSOLVE II trial met its primary endpoint: superiority of MiStent SES in minimizing in-stent late lumen loss (LLL) at nine months as compared to Medtronic's Endeavor Sprint DES (p<0.001). The trial was a randomized, multicenter study of 184 patients with documented stable or unstable angina pectoris. At nine months' follow-up, in-stent LLL was 0.27 mm with a target lesion revascularization rate of 0.9 percent. The major adverse cardiac events (MACE) rates were 4.3 percent for MiStent SES and 6.7 percent for Endeavor. In a subgroup of patients, optical coherence tomography (OCT) and endothelial function testing confirmed good vessel healing with excellent strut coverage and normal endothelial function.
The DESSOLVE I first-in-human study provides additional evidence for the potential clinical advantages of MiStent SES's unique features, as indicated by serial angiographic, intravascular ultrasound (IVUS) and OCT assessment of patients at early (6/8 month) and late (18 month) time points. Data analysis of the groups using matched pairs shows no progression of LLL (0.10/0.09 mm and 0.09 mm respectively).
Patrick W. Serruys, M.D., Ph.D., professor of interventional cardiology at the Erasmus University, Rotterdam, the Netherlands, said, "MiStent SES uniquely offers physicians an effective DES that converts to a bare metal stent in 45-60 days while still providing drug for suppression of neointimal hyperplasia up to 9 months. The MiStent SES brings us a step closer to the ideal DES, which may provide long-term efficacy while still allowing normal vessel healing."
The company is preparing for a post-marketing clinical program of 2,000 patients comparing the MiStent SES to the Xience everolimus-eluting coronary stent system in a randomized design to show non-inferiority of target lesion failure at 12 months and superior performance by the MiStent SES at 24 months with significantly less progression of LLL.
With this CE mark approval, Micell is preparing to make the MiStent SES commercially available in Europe and other markets. The MiStent SES is not currently available for sale in any market.
For more information: www.micell.com

 May 30, 2024
May 30, 2024