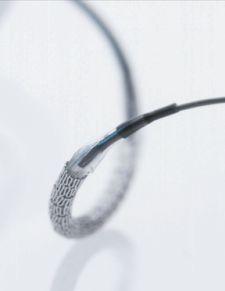
Medtronic’s Endeavor Zotarolimus-Eluting Coronary Stent System is engineered for the treatment of coronary artery disease. Endeavor uses the Driver bare metal cobalt alloy stent platform and the drug zotarolimus along with the proprietary, biocompatible drug delivery polymer.
The ENDEAVOR clinical program includes seven studies: three randomized controlled trials and four registries. Medtronic’s FDA submission included clinical data on more than 2,100 patients treated with the Endeavor stent, 1,287 of whom were studied for two years and 675 for three years.
The Endeavor was FDA-cleared in the spring of 2008.


 November 24, 2025
November 24, 2025 









