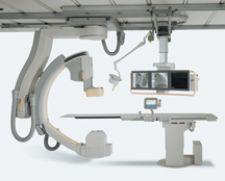
The Philips integrated cath lab is a solution featuring integrated technologies designed to improve productivity and throughput.
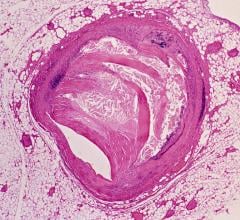
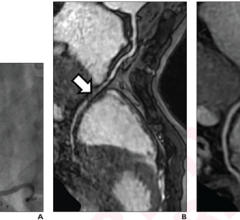
The first component is the Allura Xper FD family of interventional X-ray systems, a suite of products that is customizable for a variety of applications and available in several configurations: the FD20 and FD10 monoplane and the FD10/10 and FD20/10 biplane systems. The interventional cardiologist can optimize the system for his or her practice. Especially for complex interventions, the Allura Xper offers superb interventional tools, such as Allura 3-D-CA, which provides an instant accurate 3-D model of the coronary arteries. The StentBoost checks the deployment of stents during the intervention. All interventional tools are fully integrated in the system and instantly available during the intervention.
The Series IV physiomonitoring and information system, which was added to the Philips product portfolio through the recent acquisition of Witt Biomedical, is the second component of the integrated cath lab. Finally, the Xcelera image and information management solution provides a seamless exchange of data and images throughout the cardiology department.


 October 24, 2025
October 24, 2025