Getty Images
June 10, 2024 — New expert consensus from the Society of Cardiovascular Computed Tomography (SCCT) reviews previously published qualitative and semi-quantitative measurements – including clinical guidelines like CAD-RADS 2.0 and disease classification scores such as the CT SYNTAX score for coronary revascularization – to create a comprehensive synopsis concerning quantitative CCTA methods.
Published in the Journal of Cardiovascular Computed Tomography (JCCT), “Standards for Quantitative Assessments by Coronary Computed Tomography Angiography (CCTA)” provides a framework for terminology, methodology and reporting of quantitative CCTA analyses to create a common language for both clinicians and researchers.
“This document provides for the first time a standardization on nomenclature for quantitative parameters derived from CCTA,” said Hector Garcia-Garcia, MD, PhD, writing group co-chair and corresponding author. “It marks a turning point in reporting of CCTA as it focuses on actual quantification of atherosclerosis.”
According to the writing group – co-led by SCCT Past President Koen Nieman, MD, PhD, MSCCT – well-described semi-quantitative scores and classifications are primarily based on visual interpretation or through categories of obstructive severity rather than exact percentage stenosis measurements.
These include Agatston scores, Segment Involvement Score and assessment of stenosis and plaque within the Coronary Artery Disease Reporting and Data System (CAD-RADS), as well as disease classification scores like CT SYNTAX for coronary revascularization and CT-RECTOR Score for chronic total occlusions (CTO).
The writing group shared that, despite these available measurements and guidelines, the field currently lacks standards for the quantitative image interpretation of and reporting of CCTA results. The scientific document was created to fill that gap and create a common language for clinicians and researchers to communicate findings for coronary analyses.
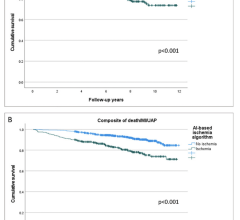
“CCTA has been recognized as a promising noninvasive tool to monitor CAD progression and assess the effects of medical therapy or mechanical interventions in clinical trials,” the group wrote. “Specific and clear definitions as well as standardization of methodology are essential for CCTA to mature as an accurate and reproducible endpoint in clinical research.”
Additional parameters covered within the document include image acquisition, quality and analysis; artifacts and image degradation; vessel and lumen segmentation; deep learning-based vessel segmentation; serial imaging studies; selection of CCTA-derived study endpoints; and future directions.
For more information: www.scct.org

 July 23, 2024
July 23, 2024