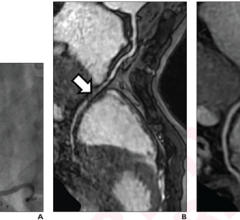
In June 2019, Medis received clearance from the U.S. Food and Drug Administration for its QAngio XA 3D technology (QFR). The FFR-angio technology can show the FFR readings in a 3-D image and overlaid on a fluoro image.
New technologies have been developed that may replace the traditional pressure wires and adenosine to assess the fractional flow reserve (FFR). This includes image-derived FFR, such as noninvasive computed tomography imaging derived FFR (FFR-CT), and X-ray angiography derived FFR (FFR-angio) that can be performed while the patient is on the table in the cath lab.
Invasive, Catheter-based FFR
Invasive FFR assessments are used in the cath lab to determine if a coronary lesion is negatively impacting hemodynamics and if stenting is required. Over the past decade, FFR has set a gold standard for quantifying the contribution of a lesion to a patient's ischemia and to justify the use of a stent, or a decision to treat a patient with drugs and monitor the lesion.
The issue with catheter-based FFR is it requires an expensive pressure wire, adds procedure time and necessitates the injection of vasodilator drugs to induce pharmacologic stress (hyperemia), usually with the drug adenosine. This adds cost and possibly time if it is sent from a central pharmacy. Adenosine also adds to the discomfort of patients. Additionally, conventional FFR only assesses a single vessel segment, so if the patient has disease in several vessels, an FFR pullback needs to be performed on each segment. These factors have limited wider use of the technology.
FFR-Angio Enters the U.S. Market
The U.S. Food and Drug Administration (FDA) cleared the first three FFR-angio software algorithms in the past year from CathWorks, Pie Imaging and Medis. All three technologies show a visual 3-D reconstruction of the coronary vessel segment and color-code the FFR readings for each section to help pinpoint culprit lesions.
Philips Healthcare also has partnered with the only FFR-CT vendor HeartFlow to develop its own version of FFR-angio. The technology uses angiographic imaging of the blood flow through the coronaries to determine FFR flow and eliminates the need for pressure wires and adenosine. This technology will likely be offered as an option on angiography imaging systems in the near future and may offer an alternative to pressures wires.
In December 2018, the FDA cleared CathWorks' FFRangio System. It derives the FFR numbers from routine X-rays acquired during a diagnostic fluoroscopic angiogram procedure. It is performed intra-procedurally during a coronary angiography, eliminating additional time and costs associated with invasive FFR. The system provides a 3-D reconstruction of the entire coronary tree with FFR values along each vessel.
The system demonstrated accuracy versus the invasive fractional flow reserve (FFR) wire in the FAST-FFR study. Read more about the trial in the article "FAST-FFR Trial of FFR-angio Technology Meets Primary Endpoint, Exceeded Performance Goals." The late-breaking FAST-FFR Trial presented last fall at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) meeting showed the sensitivity and specificity of the CathWorks FFR-angio technology matched the performance of wire-based FFR measurements. The sensitivity and specificity were 93.5 and 91.2 percent, respectively, both of which exceeded the trial’s pre-specified performance goals. The diagnostic accuracy was 92 percent overall, and remained high when considering only FFR values in the critical zone between 0.75 and 0.85. The results were simultaneously published in Circulation. [1]
CathWorks also gained approval for a new Current Procedural Terminology (CPT) code, 0523T, for FFRangio in July 2018.
Watch the VIDEO: Angiography Image-based FFR May Eliminate Need for Pressure Wires — Interview with William Fearon, M.D.
Pie Medical Imaging also has an FDA-cleared FFR-angio software called CAAS vFFR (Cardiovascular Angiographic Analysis Systems for vessel Fractional Flow Reserve). It can calculate the pressure drop and vFFR value in the coronary artery non-invasively, which means there is no need for a pressure wire and hyperemic agent.
In June 2019, Medis received clearance from the FDA for its QAngio XA 3D technology (QFR).
“Image-based QFR can quickly and efficiently help clinicians non-invasively determine whether or not they need to perform angioplasty or stenting in 5 minutes or less,” said Prof. Hans Reiber, Ph.D., CSO at Medis. “This is significantly quicker than traditional wire-based FFR procedures that can take 10-15 minutes, are invasive and require the use of a hyperemic drug, making the procedure very demanding on a patient.”
QFR is based on standard X-ray angiographic images and direct coronary flow estimation, and allows for fast in-procedure results. This software solution is designed to be X-ray vendor-independent, and to be used on both biplane and monoplane X-ray angiographic imaging systems. Image selection is facilitated through an angiographic acquisition guide and the total analysis time is typically 4-5 minutes including image frame selection.
FFR-CT Offers a Noninvasive FFR Assessment for Chest Pain
One of the big trends in cardiac computed tomography (CT) imaging has been the introduction of noninvasive FFR-CT, which can be used instead of a catheter-based FFR. This technology enables combined anatomic and hemodynamic assessment of a coronary lesion, and might be able to eliminate the need for invasive catheter-based diagnostic angiograms. HeartFlow gained FDA clearance for this technology in November 2014.
When compared with invasive FFR, CT-FFR may be “very accurate” when patients are clearly normal or abnormal, said Todd Villines, M.D., FACC, FAHA, SCCT, professor of medicine, University of Virginia Health System, and former president of the Society of Cardiovascular Computed Tomography (SCCT). “Probably its biggest limitation clinically is just using it in a binary fashion like we do invasive FFR. There is probably a wider gray zone,” he said. It is when they enter the gray zone that the cutoffs used to assess patients with invasive FFR may not apply. For example, cardiologists who assume that every CT-FFR above 0.8 indicates “normal” are in danger of error, he said. For example, values of 0.81, 0.82 or 0.83 might not have the same weight as metrics delivered by invasive FFR.
“What you would use as a cutoff for abnormal FFR in the cath lab, might not apply to CT-FFR,” Villines explained. "CT-FFR is an exciting technology that I think can serve to enhance the diagnostic yield of coronary CT.”
CT-FFR should be used selectively. “I probably wouldn’t recommend performing CT-FFR in very distal vessels, lesions in small branch vessels — lesions where medical therapy is preferred anyway,” Villines said.
Villines urged cardiologists to “be good stewards,” noting that the current (high) cost of CT-derived FFR “warrants us to look at the evidence critically.” He said published Medicare data put the cost of CT-FFR at $1,450 compared to less than $400 for invasive coronary angiography.
Although the cost differential is more than three-fold, CT-FFR might “spare someone going to the cath lab and save them from an unnecessary test or revascularization,” Villines said. “But I also think you have to counter that with being careful of overutilization.” He suggested research be conducted to assess whether the CT-based estimate is a good investment.
Villines described CT-FFR as a disruptive technology, but noted it has limited clinical value. Caution must be exercised, he said, in that its results need to be carefully considered along with the patient’s symptoms. Its clinical value depends heavily on image quality, which large studies have often found lacking. Studies comparing invasive coronary FFR to that derived from CT have rejected as many as 25 percent of CT scans due to inadequate image quality, he said.
Another limitation of FFR-CT is that the images have to be sent out for analysis. “I think one of the frustrations with a lot of clinicians about the current HeartFlow CT-FFR is that you have to send your data off-site and it takes several hours to get the answer back,” Villines explained.
However, that could change. He said FFR-CT may become more attractive if smart algorithms now in development can do the calculations on-site. He said this may allow a lower cost and a more efficient way of processing the data.
HeartFlow is testing a new percutaneous coronary intervention (PCI) planning software that allows the virtual placement of stents in lesions in the FFR-CT models created from patient scans. The software will then show the virtual impact of the stent on hemodynamic flow. At the EuroPCR Conference in May, results of the BOWIE (Benefits of Obtaining information for planning With noninvasive FFRCT prior to Invasive Evaluation) study were presented. It demonstrated that use of the HeartFlow Planner software led to a change in treatment strategy in 45 percent of patients with coronary artery disease (CAD) and reduced the need for invasive FFR physiology testing. BOWIE is a retrospective study that included 101 patients. Three interventional cardiologists independently reviewed the diagnostic angiogram for each patient and provided a treatment recommendation. The same three interventional cardiologists then reviewed each patients’ HeartFlow Analysis and used the HeartFlow Planner to virtually explore different treatment scenarios and provide a treatment recommendation. The study compared the differences in recommended treatment plans.
PODCAST: Shortcomings of CTA in Cardiac Assessements — Interview with Todd Villines, M.D.
VIDEO: Trends and Advances in Cardiac CT Technology — Interview with Todd Villines, M.D.
ARTICLE: FFR-CT: Is It Radiology or Cardiology?
Catheter Based FFR Without Adenosine and Co-registered on Fluoro
There are now two FDA-cleared FFR technologies that do not require the injection of vasodilator drugs to induce pharmacologic stress. These include Philips Volcano's instantaneous wave-free ratio (iFR), and Abbott's Resting Full-cycle Ratio (RFR).
iFR is a simplified, hyperemia-free physiological assessment of coronary blockages. Since it eliminates the need for adenosine, it can help cut costs and time to perform the diagnostic test. The technology was cleared by the FDA in April 2019.
The technology was recently updated with the SyncVision iFR co-registration product, which maps the pressure readings onto angiograms, allowing interventionalists to see where in the artery the lesion is likely located. Markers are placed along the vessel to show pressure drops to better pinpoint culprit lesions and help plan stent length and placement with a virtual stent. The software also can predict physiologic improvement with virtual stent placements with expected virtual iFR readings.
"Having access to iFR really helps make the workflow in the cath lab both efficient and economical," said John Messenger, M.D., FACC, FSCAI, director of the cardiac cath labs and director of the cardiovascular ICU/step down unit, University of Colorado Hospital. "You want to avoid the use of adenosine to induce hyperemia if you can, because it is incredibly expensive, adding cost to the case. So, if you have a clearly abnormal, intermediate stenosis assessed with an iFR catheter, that really saves us time and allows us to move forward in the case, and even do an intervention using that iFR wire."
He said having direct integration with the FFR and the fluoroscopy imaging system has been "a huge plus" because it offers adjunctive tools for faster analysis of the lesion.
Abbott's RFR was cleared by the FDA in March 2019 and also eliminates the need for adenosine.
“Abbott’s RFR provides interventionalists with a new, effective method of analysis that identifies important stenoses with the least discomfort, offering a better experience for patients," said Ziad A. Ali, M.D., director of intravascular imaging and physiology at Columbia University Medical Center's Center for Interventional Vascular Therapy.
Late-breaking trial results from the RE-VALIDATE study, an all-comers prospective analysis of retrospective real-world hospital data, was presented at the Cardiovascular Research Technologies (CRT) 2019 conference. RFR demonstrated equivalence against iFR, with overall diagnostic accuracy of 97.8 percent.[2]
Related FFR Content:
New Technology Directions in Fractional Flow Reserve (FFR)
FDA Clears Abbott's New FFR Coronary Lesion Assessment Technology
VIDEO: iFR Equal to FFR Outcomes in Coronary Lesion Evaluation — Interview with Manesh Patel, M.D.
VIDEO: DEFINE-FLAIR and SWED-HEART iFR vs. FFR trials
iFR More Cost-Effective Than FFR in PCI Guidance
Easier to Use iFR Equal to Outcomes of FFR in Coronary Lesion Evaluation
New Technology Directions in Fractional Flow Reserve (FFR)
European Society of Cardiology Incorporates iFR Technology Into Updated Revascularization Guidelines
References:



 October 24, 2025
October 24, 2025