June 4, 2019 — Medis Medical Imaging Systems B.V. has received clearance from the U.S. Food and Drug Administration for its QAangio XA 3D technology (QFR). The technology offers a non-invasive imaging technique for the assessment of the functional significance of coronary lesions without the need for a pressure wire, nor adenosine.
Coronary artery disease (CAD) develops when the coronary arteries narrow, reducing blood flow to the heart, resulting in angina (chest pain), myocardial infarction (heart attack) or death. When diagnosing suspected CAD, there is no room for doubt.
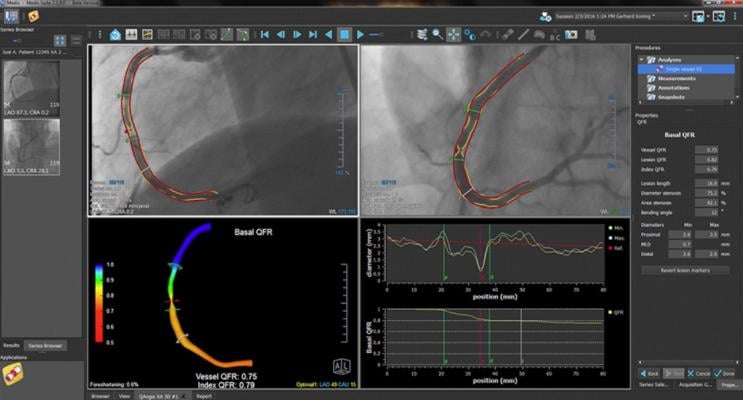
“Image-based QFR can quickly and efficiently help clinicians non-invasively determine whether or not they need to perform angioplasty or stenting in 5 minutes or less,” said Prof. Hans Reiber, PhD., CSO at Medis. “This is significantly quicker than traditional wire-based FFR procedures that can take 10-15 minutes, are invasive and require the use of a hyperemic drug, making the procedure very demanding on a patient.”
QFR is based on standard X-ray angiographic images and direct coronary flow estimation, and allows for fast in-procedure results. This software solution is designed to be X-ray vendor-independent, and to be used on both biplane and monoplane X-ray angiographic imaging systems. Image selection is facilitated through an angiographic acquisition guide and the total analysis time is typically 4-5 minutes including image frame selection.
By using QAngio XA 3D, clinicians can significantly reduce stent overuse and associated risks, better determine the correct stent length and landing zones, and help establish optimal viewing angles for stent positioning. The results are better for the patient and the clinician, according to the company.
For more information: www.medis.nl


 October 24, 2025
October 24, 2025