May 22 2018 — Pie Medical Imaging announced that clinical data on its CAAS vFFR (Cardiovascular Angiographic Analysis Systems for vessel Fractional Flow Reserve) software will be presented during EuroPCR 2018, May 22-25 in Paris, France. This software, which has received U.S. Food and Drug Administration (FDA) 510(k) clearance, can calculate the pressure drop and vFFR value in the coronary artery non-invasively, which means there is no need for a pressure wire and hyperemic agent.
FFR is an established technique used in interventional cardiology to measure pressure differences across a coronary stenosis. Based on this, cardiologists may take a decision on whether a coronary stenosis has to be treated with angioplasty or not. This examination is done during a catheterization procedure with the support of costly pressure wires and hyperemic agents.
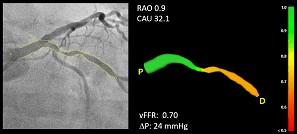
CAAS vFFR allows clinicians to use two standard angiograms taken during a catheterization procedure as input to get access to coronary physiology assessment. For percutaneous coronary interventions (PCI), within one easy workflow, CAAS vFFR offers a combination of functional and anatomical lesion assessment (such as percentage stenosis) to support the interventional cardiologist in the clinical decision making process.
The FAST study — led by Ken Masdjedi, M.D., and Joost Daemen M.D., Ph.D., from Erasmus Medical Center, Rotterdam, the Netherlands — shows that vFFR as calculated using CAAS vFFR has a high linear correlation to invasively measured FFR.
"In the FAST study, we demonstrated that vFFR as calculated using CAAS vFFR has a high linear correlation to invasively measured FFR and high diagnostic accuracy to detect FFR less than or equal to 0.80. vFFR is a promising, fast and easy to use tool to assess coronary physiology without the need for a costly pressure wire or hyperemic agent,” said Daemen, principal investigator.
CAAS vFFR is CE marked in Europe and PMDA-cleared in Japan.
For more information: www.piemedicalimaging.com


 October 24, 2025
October 24, 2025