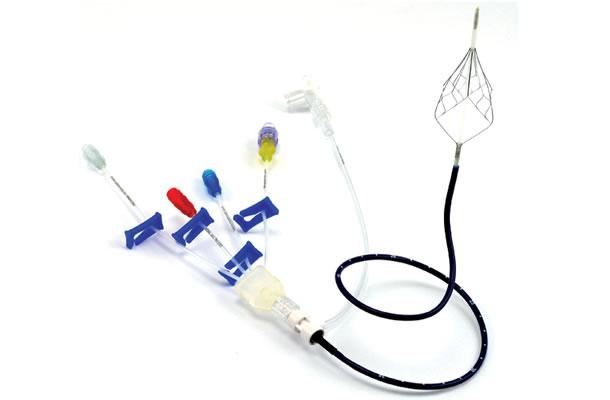
June 28, 2012 — BiO2 Medical Inc., a Texas based medical device manufacturer with corporate offices in San Antonio, Texas, and R&D and manufacturing operations in Golden, Colo., announced today that it has received CE mark approval for the Angel catheter, a nitinol inferior vena cava (IVC) filter, permanently attached to a central venous catheter (CVC) for the use of preventing pulmonary embolism (PE) in critically ill patients. This novel IVC filter/CVC combination device is the first IVC filter with a prophylactic use indication, and allows attending physicians the ability to place an IVC filter bedside in the Intensive Care Unit (ICU), in a procedure resembling a routine CVC placement procedure.
For more information: www.bio2medical.com


 April 25, 2023
April 25, 2023 









